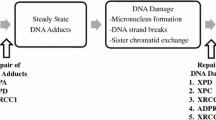
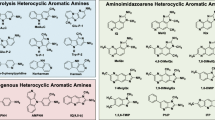
Although the etiology of colon cancer remains uncertain, an increasing body of epidemiologic evidence indicates that red meat consumption is an important risk factor. The cooking of red meat produces a class of potent experimental carcinogens, the heterocyclic aromatic amines (HAA). These induce cancers in several different sites, including the colon, in rats and mice. Other epidemiologic studies indicate that an individual's genetically determined metabolic phenotype (polymorphisms for N-acetyltransferase and N-hydroxylase) modulates the risk of colon cancer. Both N-acetyltransferase and N-hydroxylase are involved in the metabolism of HAA. An increased risk of colon cancer has been observed in rapid acetylators in four of five studies; further, in two of these the association was found only in meat eaters. The latter observation supports the hypothesis that HAA are involved in colon carcinogenesis. Considerable progress has been made in the study of the molecular pathogenesis of colon cancer, which typically entails the cumulation of several genetic events (mutations and deletions) in oncogenes and tumor suppressor genes. It would now be a crucial contribution to elucidating the causation of colon cancer to show that such mutations are induced in human colonic mucosa by food-borne heterocyclic aromatic amines.
Similar content being viewed by others
References
McKeown-Eyssen GE, Bright-See E. Dietary factors in colon cancer: International relationships. Nutrition and Cancer 1984; 6: 160–70.
Haenszel W, Berg JW, Segi M, et al. Large bowel cancer in Hawaiian Japanese. JNCI 1973; 51: 1765–79.
Bjelke R. Epidemiologic studies of cancer of the stomach, colon, and rectum; with special emphasis on the role of diet. Scand J Gastroenterol 1974; 31 (suppl): 124–229.
Dales LG, Friedman GD, Ury HK, et al. A case-control study of relationships of diet and other traits to colorectal cancer in American blacks. Am J Epidemiol 1979; 109: 132–44.
Miller AB, Howe GR, Jain M, et al. Food items and food groups as risk factors in a case-control study of diet and colorectal cancer. Int J Cancer 1983; 32: 155–61.
Manousos O, Day NE, Trichopoulos D, et al. Diet and colorectal cancer: a case-control study in Greece. Int J Cancer 1983; 32: 1–5.
Kune S, Kune GM, Watson F. Case-control study of dietary etiologic factors: the Melbourne Colorectal Cancer Study. Nutr Cancer 1987; 9: 21–42.
Young TB, Wolf DA. Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer 1988; 42: 167–75.
LaVecchia C, Negri E, Decarli A, et al. A case-control study of diet and colorectal cancer in northern Italy. Int J Cancer 1988; 41: 492–8.
Benito E, Stiggelbout A, Bosch X, et al. Nutritional factors in colorectal cancer risk: a case-control study in Majorca. Int J Cancer 1991; 49: 161–7.
Peters RK, Pike MC, Garabrant D, et al. Diet and colon cancer in Los Angeles County, California. Cancer Causes Control 1992; 3: 457–73.
Bidoli E, Franceschi S, Talamini R, et al. Food consumption and cancer of the colon and rectum in north-eastern Italy. Int J Cancer 1992; 50: 223–9.
Schiffman MH, Felton JS. Fried foods and the risk of colon cancer. Am J Epidemiol 1990; 131: 376–8.
Gerhardsson DeVerdier M, Hagman U, Steineck G, Rieger A, Norell SE. Diet, body mass and colorectal cancer: a case-referent study in Stockholm. Int J Cancer 1990; 46: 832–8.
Wohlleb JC, Hunter CF, Blass B, Kadlubar FF, Chu DZJ, Lang NP. Aromatic amine acetyltransferase as a marker for colorectal cancer: Environmental and demographic associations. Int J Cancer 1990; 46: 22–30.
Lang NP, Butler MA, Massengill J, et al. Rapid metabolic phenotypes for Acetyltansferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer or polyps. Cancer Epidemiol Biomark Prev 1994; 3: 675–82.
Roberts-Thomson IC, Ryan P, Khoo KK, Hart WJ, McMichael AJ, Butler RN. Diet, acetylator phenotype and risk of colorectal neoplasia. Lancet (in press).
Willett WC, Stampfer MJ, Colditz GA, et al. Relation of meat, fat and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med 1990; 323: 1164–72.
Giovannucci E, Rimm ER, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res 1994; 54: 2390–7.
Phillips RL, Snowdon DA. Dietary relationships with fatal colorectal cancer among Seventh-Day Adventists. JNCI 1985; 74: 307–17.
Goldbohm RA, van denBrandt PA, van't Veer P, et al. A prospective cohort study on the relation between meat consumption and the risk of colon cancer. Cancer Res 1994; 54: 718–23.
Knekt P, Steineck G, Jarvinen R, Hakulinen T, Aromaa A. Intake of fried meat and risk of cancer: a follow-up study in Finland. Int J Cancer 1994; 59: 756–60.
Bostick RM, Potter JD, Kushi LH, et al. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States). Cancer Causes Control 1994; 5: 38–52.
Ohgaki H, Kusama K, Matsukura N, et al. Carcinogenicity in mice of a mutagenic compound, 2-amino-3-methylimidazo (4,5-f) quinoline, from broiled sardine, cooked beef and beef extract. Carcinogenesis 1984; 5: 921–4.
Ohgaki H, Hasegawa H, Suenaga M, et al. Induction of hepatocellular carcinoma and highly metastatic squamous cell carcinomas in the forestomach of mice by feeding 2-amino-3,4-dimethylimidazo (4,5-f) quinoline. Carcinogenesis 1986; 7: 1889–93.
Pariza MW, Aesehbacher HU, Felton JS, Sato S. Mutagens and Carcinogens in the Diet. New York, NY (USA): 1990. Wiley-Liss.
Bogen KT. Cancer potencies of heterocyclic amines found in cooked foods. Fd Chem Toxic 1994; 32: 505–15.
Pfau W, Brockstedt U, Marquardt H. HPLC analysis of p32-labelled DNA adducts formed by polycyclic aromatic hydrocarbons, polycyclic aromatic amines or heterocyclic aromatic amines. Proceedings, Risk Assessment in Environmental Carcinogenesis, Whistler, Canada, 17–22 January 1994.
Friesen M, Kaderik K, Lin D, et al. Analysis of DNA adducts of 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine in rat and human tissues by alkaline hydrolysis and gas chromatography/electron capture mass spectrometry: validation by comparison with 23P-postlabeling. Chem Res Toxicol 1994; 7: 733–9.
Turesky RJ, Lang NP, Butler MA, et al. Metabolic activation of carcinogenic heterocyclic aromatic amines by human liver and colon. Carcinogenesis 1991; 12: 1839–45.
Felton JS, Knize MG. Occurrence, identification and bacterial mutagenicity of heterocyclic amines in cooked food. Mutat Res 1991; 259: 205–17.
International Agency for Research on Cancer. Polynuclear Aromatic Compounds, Part 1. Lyon, France: IARC 1993; IARC Monogr Eval Carcinog Risk Chem Humans, Vol. 56: 229–242.
Turteltaub KW, Creek MR, Frantz C, Markee C, Felton JS. Low dose effects on pharmacokinetics and DNA adduction for the carcinogens benzene and MeIQx in rodents: implications for risk assessment. Proceedings, Risk Assessment in Environmental MI; Carcinogenesis, Whistler, Canada, 17–22 January 1994.
Ushiyama H, Wakabayashi K, Hirose M, Itoh H, Sugimura T, Nagao M. Presence of carcinogenic heterocyclic amines in urine of healthy volunteers eating normal diet, but not in patients receiving parenteral alimentation. Carcinogenesis 1991; 12: 1417–22.
Ji H, Yu MC, Stillwell WG, et al. Urinary excretion of 2-amino-3,8-dimethylimidazo-(4,5-f)-quinoxaline in white, black and Asian men in Los Angeles County. Cancer Epidemiol Biomark Prev 1994; 3: 407–11.
Layton DW, Bogen KT, Knize MG, Hatch FT, Johnson VM, Felton JS. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis 1995; 16: 39–52.
Butler MA, Lang NP, Young JF, et al. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics 1992; 2: 116–27.
Lang NP, Chu DZJ, Hunter CF, Kendall DC, Flammang TJ, Kadlubar FF. Role of aromatic amine acetyltransferase in human colorectal cancer. Arch Surg 1986; 121: 1259–61.
Ilett KF, David BM, Dethon P, Castelden WM, Kwa R. Acetylation phenotype in colorectal carcinoma. Cancer Res 1987; 47: 1466–9.
Ladero JM, Gonzalez JF, Benitez J, et al. Acetylation polymorphism in human colorectal carcinoma. Cancer Res 1991; 51: 2098–100.
Lang NP, Butler MA, Massengill J, Lawson M, Kadlubar FF. Aromatic amine acetylation and N-oxidation phenotype in colorectal cancer, polyp and control patients. Proceedings, Fifth International Conference on Carcinogenic and Mutagenic N-substituted Aryl Compounds. Wurzburg, Germany, October 18–21, 1992.
Probst-Hensch NM, Haile RW, Ingles SA, et al. Acetylation polymorphism and prevalence of colorectal carcinoma. Cancer Res 1995; 55: 2017–20.
Shibuta K, Nakashima T, Abe M, et al. Molecular genotyping for N-acetylation polymorphism in Japanese patients with colorectal cancer. Cancer 1994; 74: 3108–12.
Oda Y, Tanaka M, Nakanishi I. Relation between the occurrence of K-ras gene point mutations and genotypes of polymorphic N-acetyltransferase in human colorectal carcinomas. Carcinogenesis 1994; 15: 1365–9.
Nerurkar PV, Schut HAJ, Anderson LM, et al. DNA adducts of 2-amino-3-methylimidazo(4,5-f)quinoline (IQ) in colon, bladder, and kidney of congenic mice in Ah responsiveness and N-acetyltransferase genotype. Cancer Res 1995; 55: 3043–9.
Nakachi K, Imai K, Hayashi S, et al. Polymorphisms of the CYP1A1 and Glutathione S-Transferase genes associated with susceptibility to lung cancer in relation to cigarette dose in a Japanese population. Cancer Res 1993; 53: 2994–9.
Vineis P, Bartsch H, Caporaso N, et al. Genetically based N-acetyltransferase metabolic polymorphism and low-level environmental exposure to carcinogens. Nature 1994; 369: 154–6.
Doll R. An epidemiological perspective of the biology of cancer. Cancer Res 1978; 38: 3573–83.
Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal tumor development. N Engl J Med 1988; 319: 525–32.
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–67.
McMichael AJ. ‘Molecular epidemiology’: new pathway or new travelling companion? Am J Epidemiol 1994; 140: 1–11.
Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutation in human cancers. Science 1991; 252: 49–53.
Beland FA, Kadlubar FF. Formation and persistence of arylamine-DNA adducts in vivo. Environ Health Persp 1985; 62: 19–30.
Author information
Authors and Affiliations
Additional information
This work has been supported in part by the Associazione Italiana per la Ricerca sul Cancro and by the Italian National Research Council (Progetto Finalizzato ACRO, Grant No. 93.04716.CT04).
Rights and permissions
About this article
Cite this article
Vineis, P., McMichael, A. Interplay between heterocyclic amines in cooked meat and metabolic phenotype in the etiology of colon cancer. Cancer Causes Control 7, 479–486 (1996). https://doi.org/10.1007/BF00052675
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00052675