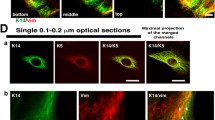
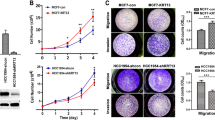
Summary
The expression of intermediate filament proteins is remarkably tissue-specific which suggests that the intermediate filament (IF) type(s) present in cells is somehow related to their biological function. However, in some cancers-particularly malignant melanoma and breast carcinoma, there is a strong indication that vimentin and keratin IFs are coexpressed, thus presenting as a dedifferentiated or interconverted (between epithelial and mesenchymal) phenotype. In this review, twoin vitro models are presented which recapitulate the interconverted phenotype in human melanoma and breast carcinoma, and allow, for the first time, unique observations to be made with respect to the role of IFs in cancer progression.
These studies have provided direct evidence linking overexpression of keratin IFs in human melanoma with increased migratory and invasive activityin vitro, which can be down-regulated by substituting dominant-negative keratin mutants. Overexpression of vimentin IFs in the breast carcinoma model leads to augmentation of motility and invasivenessin vitro, which can be transiently down-regulated by treatment with antisense oligonucleotides to vimentin. Additional experimental evidence suggests that the mechanism(s) responsible for the differential expression of metastatic properties associated with the interconverted phenotype rest(s) in the unique interaction, either direct or indirect, of IFs with specific integrins interacting with the extracellular matrix.
In this review, we discuss the observations derived from the human melanoma and breast carcinoma models to address the hypothesis that the ability to coexpress vimentin and keratins confers a selective advantage to tumor cells in their interpretation of and response to signaling cues from the extracellular matrix. The ramifications of these observations are discussed with respect to the patholophysiology of the respectivein situ tumors.
Similar content being viewed by others
References
Rigel D, Kopf AW, Friedman RJ: The rate of malignant melanoma in the United States: Are we making an impact? J Am Acad Dermatol 17: 1050–1053, 1981
Raymond WA, Leong AS-Y: Vimentin — A new prognostic parameter in breast carcinoma? J Pathol 158: 107–114, 1989
Akslen LA, Hove LM, Harveit F: Metastatic distribution in malignant melanoma: A 30-year autopsy study. Invasion Metas 7: 253–263, 1987
Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell 64: 327–336, 1991
Kohn EC, Liotta LA: Molecular insights into cancer invasion: Strategies for prevention and intervention. Cancer Res 55: 1856–1862, 1995
Huszar M, Halkin H, Herczeg E, Bubis J, Geiger B: Use of antibodies to intermediate filaments in the diagnosis of metastatic melanoma. Human Pathol 14: 1006–1008, 1983
Osborn M, Weber K, Biology of disease: Tumor diagnosis by intermediate filament typing — A new tool for surgical pathology. Lab Invest 48: 372–394, 1983
Steinert PM, Leim RK: Intermediate filament dynamics. Cell 60: 521–523, 1990
Klymkowsky MW, Bachant JB, Domingo A: Functions of intermediate filaments. Cell Motility Cytoskel 14: 309–331, 1989
Skalli O, Goldman RD: Recent insights into the assembly, dynamics, and function of intermediate filament networks. Cell Motility Cytoskel 19: 67–79, 1991
Fuchs E, Weber K: Intermediate filaments: Structure, dynamics, function and disease. Annu Rev Biochem 63: 345–382, 1994
Osborn M, Weber K: Intermediate filaments: Cell-type-specific markers in differentiation and pathology. Cell 31: 303–306, 1982
Franke WW, Schmid E, Moll R: The intermediate filament cytoskeleton in tissues and in cultured cells: Differentiation specificity of expression of cell architectural elements. In: Harris CC, Autrup JN (eds) Human carcinogenesis, New York: Academic Press, 1983, pp 3–34
Hendrix MJC, Seftor EA, Chu Y-W, Seftor REB, Nagle RB, McDaniel KM, Leong SPL, Yohem KH, Leibovitz A, Meyskens FL, Conoway DH, Welch DR, Liotta LA, Stetler-Stevenson WG: Coexpression of vimentin and keratins by human melanoma tumor cells: Correlation with invasive and metastatic potential. J Natl Cancer Inst 84: 165–174, 1992
Miettinen M, Franssila K: Immunohistochemical spectrum of malignant melanoma: The common presence of keratins. Lab Invest 61: 623–628, 1989
Zarbo RJ, Grown AM, Nagle RB: Anomalous cytokeratin expression in malignant melanoma: One-and two-dimensional Western blot analysis and immunohistochemical survey of 100 melanomas. Mod Pathol 3: 494–501, 1990
Thompson EW, Soonmyoung P, Brunner N, Sommers CL, Sugmaler G, Clarke R, Shima TB, Torri J, Donahue S, Lippmann ME, Martin GR, Dickson RB: Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol 150: 534–544, 1992
Cattoretti G, Andreola S, Clemente C, D'Amato L, Rilke F: Vimentin and p53 expression on EGFr-positive, estrogen receptor-negative breast carcinomas. Br J Cancer 57: 353–357, 1988
Sommers CL, Heckford SE, Skerker JM, Worland P, Torri JA, Thompson EW, Byers SW, Gelmann EP: Loss of epithelial markers and acquisition of vimentin expression in adriamycin- and vinblastine-resistant human breast cancer cell lines. Cancer Res 52: 5190–5197, 1992
Sommers CL, Byers SW, Thompson EW, Torri JA, Gelmann EP: Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat 31: 325–335, 1994
Bianchi E, Cohen RL, Dai A, Thor AD, Shuman MA, Smith HA: Immunohistochemical localization of the plasminogen activator inhibitor-1 in breast cancer. Int J Cancer 60: 597–603, 1995
Ben-Ze'ev A, Zoller M, Raz A: Differential expression of intermediate filament proteins in metastatic and nonmetastatic variants of the Bsp73 tumor. Cancer Res 46: 785–790, 1986
Ramaekers FCS, Haag D, Kant A, Moesker O, Jap PHK, Vooljs GP: Coexpression of keratin- and vimentin-type intermediate filaments in human metastatic carcinoma cells. Proc Natl Acad Sci USA 80: 2618–2622, 1983
Gunther A, Kinjo M, Winter H, Sonka J, Volm D: Differential expression of intermediate-filament proteins in murine sarcoma 180 ascites of solid tumor. Cancer Res 44: 2590–2594, 1984
Kinjo M, Winter H, Schwelzer J: Differential expression of intermediate filament proteins in two rat ascites hepatoma lines of common origin. Carcinogenesis 5: 1249–1255, 1984
Stewart M: Intermediate filaments: Structure, assembly and molecular interactions. Current Opin Cell Biol 12: 91–100, 1994
Florenes VA, Holm R, Myklebost O, Lendahl U, Fodstad O: Expression of the neuroectodermal intermediate filament nestin in human melanomas. Cancer Res 54: 354–356, 1994
Fuchs E, Marchuk D: Type I and type II keratins have evolved from lower eukaryotes to form the epidermal intermediate filaments in mammalian skin. Proc Natl Acad Sci USA 80: 5857–5861, 1983
Steinert PM: The two-chain coiled-coil molecule of native epidermal keratin intermediate filaments is a type I-type II heterodimer. J Biol Chem 265: 8766–8774, 1990
Fuchs E: Epidermal differentiation: The bare essentials. J Cell Biol 111: 2807–2814, 1990
Oshima RG, Howe WE, Klier FG, Adamson ED, Shevinsky LH: Intermediate filament protein synthesis in preimplantation murine embryos. Dev Biol 99: 447–455, 1983
Duprey P, Morello D, Vasseur M, Babinet C, Condamine H, Brulet P, Jacob F: Expression of the cytokeratin endo A gene during early mouse embryogenesis. Proc Natl Acad Sci USA 82: 8535–8539, 1985
Lane EB, Hogan BLM, Kurkinen M, Garrels JI: Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature 303: 701–703, 1983
Trejdosiewicz LK, Southgate J, Kemshead JT: Phenotypic analysis of cultured melanoma cells: Expression of cytokeratin-type intermediate filaments by the M5 human melanoma cell line. Exp Cell Res 164: 388–398, 1986
Rosen EM, Nigam SK, Goldberg ID: Scatter factor and the c-met receptor: A paradigm for mesenchymal/epithelial interaction. J Cell Biol 127: 1783–1787, 1994
Tyner AL, Fuchs E: Evidence for posttranslation regulation of the keratins expressed during hyperproliferation and malignant transformation in human epidermis. J Cell Biol 103: 1945–1955, 1986
Knapp AC, Franke WW: Spontaneous losses of control of cytokeratin gene expression in transformed, non-epithelial human cells occurring at different levels of regulation. Cell 59: 67–79, 1989
Oshima RG, Abrams L, Kulesh D: Activation of an intron enhancer within the keratin 18 gene by expression of c-fos and c-jun in undifferentiated F9 embryonal carcinoma cells. Genes Dev 4: 835–848, 1990
Oshima RG: Intermediate filament molecular biology. Curr Opin Cell Biol 4: 110–116, 1992
Fuchs E: Intermediate filaments and disease: Mutations that cripple cell strength. J Cell Biol 125: 511–516, 1994
Hay ED: Theory for epithelial-mesenchymal transformation based on the ‘fixed cortex’ cell motility model. Cell Motil Cytoskeleton 14: 455–457, 1989
McDonald JA: Matrix regulation of cell shape and gene expression. Curr Opin Cell Biol 1: 995–999, 1989
Steuli CH, Bissell MJ: Mammary epithelial cells, extracellular matrix and gene expression. Cancer Treat Res 53: 365–381, 1991
Juliano RL, Haskill S: Signal transduction from the extracellular matrix. J Cell Biol 120: 577–585, 1993
Ginsberg MH, Du X, Plow EF: Inside-out integrin signaling. Curr Opin Cell Biol 4: 766–771, 1992
Ingber D: Integrins as mechanochemical transducers. Curr Opin Cell Biol 3: 841–848, 1991
Akiyama SK, Nagata K, Yamada KM: Cell surface receptors for extracellular matrix components. Biochem Biophys Acta 103: 91–109, 1990
Albelda SM, Buck CA: Integrins and other cell adhesion molecules. FASEB J 4: 2868–2880, 1990
Ruoslahti E: Integrins. J Clin Invest 87: 1–5, 1991
Matrisian M: Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 6: 121–125, 1990
Mignatti P, Rifkin DB: Biology and biochemistry of proteinases in tumor invasion. Phys Rev 73: 161–195, 1993
Sloane BF: Cathepsin B and cystatins: Evidence for a role in cancer progression. Semin Cancer Biol 1: 137–152, 1990
Bernhard EJ, Gruber SB, Muschel RJ: Direct evidence linking the expression of MMP-9 to the transformed phenotype in transformed rat cells. Proc Natl Acad Sci USA, 1994
Liotta LA, Stetler-Stevenson WG: Metalloproteinases and cancer invasion. Semin Cancer Biol 1: 99–106, 1990
Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH: Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol 109: 877–889, 1989
Seftor REB, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJC: Role of the αvβ3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA 89: 157–1561, 1992
Seftor REB, Seftor EA, Stetler-Stevenson WG, Hendrix MJC: The 72 kDa type IV collagenase is modulated via differential expression of the αvβ3 and α5β1 integrins during human melanoma cell invasion. Cancer Res 53: 3411–3415, 1993
Quaranta V, Jones JCR: The internal affairs of an integrin. Trends Cell Biol 1: 2–4, 1991
Wayner EA, Orlando RA, Cheresh DA: Integrins αvβ3 and αvβ5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol 113: 919–929, 1991
Tawil N, Wilson P, Carbonetto S: Integrins in point contacts mediate spreading. J Cell Biol 120: 261–271, 1993
Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM: Integrin function: Molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol 131: 791–805, 1995
Green KJ, Goldman RD: Evidence for an interaction between the cell surface and intermediate filaments in cultured fibroblasts. Cell Motil Cyto 6: 389–405, 1986
Otey CA, Pavalko FM, Burridge K: An interaction between α-actinin and the β1 integrin subunitin vitro. J Cell Biol 111: 721–729, 1990
Chu Y-W, Seftor EA, Romer LH, Hendrix MJC: Experimental coexpression of vimentin and keratin intermediate filaments in human melanoma cells augments motility. Am J Pathol 148: 63–69, 1996
Hynes RO: Integrins: Versatility, modulation and signaling in cell adhesion. Cell 69: 11–25, 1992
Ruoslahti E: Control of cell motility and tumor invasion by extracellular matrix interactions. Br J Cancer 66: 239–242, 1992
Kornberg LJ, Earp HS, Turner CE, Prockop C, Juliano RL: Signal transduction by integrins: Increased protein tyrosine phosphorylation caused by clustering of α1 integrins. Proc Natl Acad Sci USA 88: 8392–8396, 1991
Romer LH, McLean N, Turner CE, Burridge K: Tyrosine kinase activity, cytoskeletal organization and motility in human vascular endothelial cells. Mol Biol Cell 5: 349–361, 1994
Nuckolls GH, Romer LH, Burridge K: Microinjection of antibodies against talin inhibits the spreading and migration of fibroblasts. J Cell Sci 102: 753–762, 1992
Busso N, Masur SK, Lazega D, Waxman S, Ossowski L: Induction of cell migration by pro-urokinase binding to its receptor. Possible mechanism for signal transduction in human epithelial cells. J Cell Biol 126: 259–270, 1994
Chou C-F, Omary MB: Mitotic-arrest associated enhancement of O-linked glycosylation and phosphorylation of human keratins 8 and 18. J Biol Chem 268: 4456–4472, 1993
Liao J, Lowthert LA, Ku N-O, Fernandex R, Omary MB: Dynamics of human keratin 18 phosphorylation: Polarized distribution of phosphorylated keratins in simple epithelial tissues. J Cell Biol 131: 1291–1301, 1995
Burridge K, Turner CE, Romer LH: Tyrosine phosphorylation of paxillin and pp125125 accompanies cell adhesion to extracellular matrix: A role in cytoskeletal assembly. J Cell Biol 119: 893–903, 1992
Kornberg LJ, Earp HS, Parsons JT, Schaller M, Juliano RL: Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem 267: 23439–23442, 1992
Weiner TM, Liu ET, Craven RJ, Cance WG: Expression of focal adhesion kinase gene and invasive cancer. Lancer 342: 1024–1025, 1993
Owens LV, Xu L-H, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG: Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res 55: 2752–2755, 1995
Chu YW, Duffy JJ, Seftor REB, Hendrix MJC: Disruption of normal cytokeratin formation by the transfection of a delected CK18 cDNA into a highly metastatic melanoma cell line decreases the invasive potential. J Clin Biotch 3: 27–33, 1991
Hendrix MJC, Seftor EA, Seftor REB, Fidler IJ: A simple quantitative assay for studying the invasive potential of high and low metastatic variants. Cancer Lett 38: 137–147, 1987
Boring CC, Squires TS, Tong T: Cancer statistics. Cancer J Clin 20: 7–26, 1993
Domagala W, Lasota J, Bartowiak J, Weber K, Osborn M: Vimentin is preferentially expressed in human breast carcinomas with low estrogen receptor and high Ki-67 growth fraction. Am J Pathol 136: 219–227, 1990
Domagala W, Leszek W, Lasota J, Weber K, Osborn M: Vimentin is preferentially expressed in high-grade ductal and medullary, but not in lobular breast carcinomas. Am J Pathol 137: 1059–1064, 1990
Thompson EW, Paik S, Brunner N, Sommers CL, Zugmaier G, Clarke R, Shima TB, Torri J, Donahue S, Lippman ME, Martin GR, Dickson RB: Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol 150: 534–544, 1992
Sommers CL, Walker-Jones D, Heckford SE, Worland P, Valverius E, Clark R, Stampfer M, Gelmann EP: Vimentin rather than keratin expression in some hormone-independent breast cancer cell lines in oncogene-transformed mammary epithelial cells. Cancer Res 49: 4258–4263, 1989
McLean WHI, Lane EB: Intermediate filaments in disease. Current Opin Cell Biol 7: 118–125, 1995
Klymkowsky MW: Intermediate filaments: new proteins, some answers, more questions: Current Opin Cell Biol 7: 46–54, 1995
McCarthy JB, Palm SL, Furcht LT: Migration by haptotaxis of a Schwann cell tumor cell line to the basement membrane glycoprotein laminin. J Cell Biol 97: 772–777, 1983
Hamburger AW, Salmon SE: Primary bioassay of human tumor stem cells. Science 197: 461–463, 1977
Stetler-Stevenson WG, Aznavoorian S, Liotta LA: Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 9: 541–573, 1993
Chu Y-W, Runyan RB, Oshima RG, Hendrix MJC: Expression of complete keratin filaments in mouse L cells augments cell migration and invasion. Proc Natl Acad Sci USA 90: 4261–4265, 1993
Sarria AJ, Nordeen SK, Evans RM: Regulated expression of vimentin cDNA in cells in the presence and absence of a preexisting vimentin filament network. J Cell Biol 111: 553–565, 1990
Trevor KT: Disruption of keratin filaments in embryonic epithelial cell types. New Biol 2: 1004–1014, 1990
Gunning P, Leavitt J, Muscat G, Ng S-Y, Kedes L: A human beta-actin expression vector system directs high levels accumulation of antisense transcripts. Proc Natl Acad Sci USA 84: 4831–4835, 1987
Gorman CM, Merlino GT, Willingham MC, Pastan I, Howard BH: The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci USA 79: 6777–6781, 1982
Andreoli JM, Trevor KT: Structural and biological consequences of increased vimentin expression in simple epithelial cell types. Cell Motil Cytoskel 32: 10–25, 1995
Bauman PA, Dalton WS, Anderson JM, Cress AE: Expression of cytokeratin cofers multiple drug resistance. Proc Natl Acad Sci USA 91: 5311–5314, 1994
Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB: Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor β3. Proc Natl Acad Sci USA 88: 1516–1520, 1991
Shea TB, Beermann ML, Fischer I: Transient requirement for vimentin in neuritogenesis: intracellular delivery of anti-vimentin antibodies and antisense oligonucleotides inhibit neurite initiation but not elongation of existing neurites in neuroblastoma. J Neurosci Res 36: 66–76, 1993
Maiorana A, Cesinaro AM, Fano RA, Collina G: Expression of MHC class I and class II antigens in primary breast carcinomas and synchronous nodal metastases. Clin Exp Metas 13: 43–48, 1995
Lichtner RB, Julian JA, North SM, Glasser SR, Nicolson GL: Coexpression of cytokeratins characteristic for myoepithelial and luminal cell lineages in rat 1376NF mammary adenocarcinoma tumors and their spontaneous metastases. Cancer Res 51: 5943–5950, 1991
Stover DM, Carey I, Garzon RJ, Zehner ZE: A negative regulatory factor is missing in a human metastatic breast cancer cell line. Cancer Res 54: 3092–3095, 1994
Griffith CM, Hay ED: Epithelial-mesenchymal transformation during palatal fusion: carboxyfluorescein traces cells at light and electron microscopic levels. Development 116: 1087–1099, 1992
Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM: Integrin function: Molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol 131: 791–805, 1995
Kobota S, Tashiro K, Yamada Y: Signaling site of laminin with mitogenic activity. J Biol Chem 257: 4285–4288, 1992
Zutter MM, Santoro SA, Staatz WD, Tsung YL: Re-expression of the α2β1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci USA 92: 7411–7415, 1995
Lynch RG: Differentiation and cancer. Proc Natl Acad USA 92: 647–648, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hendrix, M.J.C., Seftor, E.A., Chu, YW. et al. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metast Rev 15, 507–525 (1996). https://doi.org/10.1007/BF00054016
Issue Date:
DOI: https://doi.org/10.1007/BF00054016