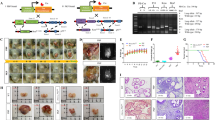
Carcinomas of the prostate and other lineages often present an autocrine stimulatory loop acting via the EGF receptor (EGFR). We have recently shown that EGFR-mediated signals enhance DU-145 prostate carcinoma cell transmigration of an extracellular matrix in vitro, and that this increased invasiveness was independent of proteolytic degradation of the matrix (Xie et al., 1995, Clin Exp Metastasis, 13, 407). To determine whether up-regulated EGFR signaling promotes tumor progression in vivo and to define the EGFR-induced cell property responsible, we inoculated athymic mice with genetically-engineered DU-145 cells. Parental DU-145 cells and those transduced to overexpress a full-length wild type (WT) EGFR formed tumors and metastasized to the lung when inoculated in the prostate and peritoneal cavity. The WT DU-145 tumors were more invasive. DU-145 cells expressing a mitogenically-active, but motility-deficient (c'973) EGFR formed small, non-invasive tumors without evidence of metastasis. All three sublines demonstrated identical, EGFR-dependent rates of cell growth in vitro, suggesting that the differential invasiveness was not due to altered growth rates. To determine whether cell motility may be, in part, responsible for tumor invasiveness, we treated WT DU-145 intraperitoneal tumors with a pharmacologic agent (U73122) which blocks EGFR-mediated cell motility but not mitogenesis. Under this treatment regimen, the WT DU-145 cells formed tumors of similar numbers and size to those formed without treatment; however, these tumors were much less invasive. These data suggest that EGFR-mediated cell motility is an important mechanism involved in tumor progression, and that this cell property may represent a novel target to limit the spread of tumors.
Similar content being viewed by others
References
Partin AW and Coffey DS, 1994, Benign and Malignant Prostatic Neoplasms: human studies. San Diego, California: Academic Press.
Sandberg AA, 1992, Cytogenetic and Molecular Genetic Aspects of Human Prostate Cancer: primary and metastatic. New York: Plenum Press.
Geldof AA and Rao BR, 1990, Factors in prostate cancer metastasis. Anticancer Res, 10, 1303–6.
Linehan WM, 1995, Inhibition of prostate cancer metastasis: a critical challenge ahead. J Natl Cancer Inst, 87, 331–2.
Gittes RF, 1991, Carcinoma of the prostate. N Engl J Med, 324, 236–45.
Surya BV and Provet JA, 1989, Manifestations of advanced prostate cancer: prognosis and treatment. J Urol, 142, 921–8.
McKeehan WL, 1986, Growth factors spawn new cell cultures. Nature, 321, 629–30.
Chaproniere DM and Webber MM, 1985, Dexamethasone and retinyl acetate similarly inhibit and stimulate EGF or insulin-induced proliferation of prostatic epithelium. J Cell Physiol, 122, 249–53.
Eaton CL, Davies P and Phillips MEA, 1988, Growth factor involvement and oncogene expression in prostatic tumors. J Ster Biochem, 30, 341–5.
Marti U, Burwen SJ and Jones AL, 1989, Biological effects of epidermal growth factor, with emphasis on the gastrointestinal tract and liver: an update. Hepatology, 9, 126–38.
Connolly JM and Rose DP, 1989, Secretion of epidermal growth factor and related polypeptides by the DU 145 human prostate cancer cell line. Prostate, 15, 177–86.
Nishi N, Matuo Y and Wada F, 1988, Partial purification of a major type of rat prostatic growth factor: characterization as an epidermal growth factor-related mitogen. Prostate, 13, 209–20.
Wilding G, Valverius E, Knabbe C and Gelmann EP, 1989, Role of transforming growth factor-α in human prostate cancer cell growth. Prostate, 15, 1–12.
Liu X-H, Wiley HS and Meikle AW, 1993, Androgens regulate proliferation of human prostate cancer cells in culture by increasing transforming gowth factor-α (TGF-α) and epidermal growth factor (EGF)/TGF-α receptor. J Clin Endocr Metab, 77, 1472–8.
Aaronson SA, 1991, Growth factors and cancer. Science, 254, 1146–53.
Schlegel J, Merdes A, Stumm G, et al., 1994, Amplification of the epidermal growth factor receptor gene correlates with different growth behavior in human glioblastoma. Int J Cancer, 56, 72–7.
Collins VP, 1993, Amplified genes in human gliomas. Sem Cancer Biol, 4, 27–32.
Yasui W, Sumiyoshi H, Hata J, et al., 1988, Expression of epidermal growth factor receptor in human gastric and colon carcinomas. Cancer Res, 48, 137–41.
Neal DE, Marsh C, Bennett MK, et al., 1985, Epidermal-growth-factor receptors in human bladder cancer: comparison of invasive and superficial tumours. Lancet, i, 366–8.
Nguyen PL, Swanson PE, Jaszcz W, et al., 1994, Expression of epidermal growth factor receptor in invasive transitional cell carcinoma of the urinary bladder: a multivariate survival analysis. Am J Clin Pathol, 101, 166–76.
Klijn JG, Berns PM, Schmitz PI and Foekens JA, 1992, The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev, 13, 3–17.
Sainsbury JRC, Farndon JR, Needham GK, Malcolm AJ and Harris AL, 1987, Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet, i, 1398–402.
Radinsky R, Risin S, Fan D, et al., 1995, Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res, 1, 19–31.
Lubrano C, Petrangeli E, Catizone A, et al., 1989, Epidermal growth factor binding and steroid receptor content in human benign prostatic hyperplasia. J Ster Biochem, 34, 499–504.
Morris GL and Dodd JG, 1990, Epidermal growth factor receptor mRNA levels in human prostatic tumors and cell lines. J Urol, 143, 1272–4.
Davies P and Eaton CL, 1989, Binding of epidermal growth factor by human normal, hypertrophic, and carcinomatous prostate. Prostate, 14, 123–32.
Myers RB, Kudlow JE and Grizzle WE, 1993, Expression of transforming growth factor alpha, epidermal growth factor and the epidermal growth factor receptor in adenocarcinoma of the prostate and benign prostatic hyperplasia. Modern Pathol, 6, 733–7.
Ching KZ, Ramsey E, Pettigrew N, D'Cunha R, Jason M and Dodd JG, 1993, Expression of mRNA for epidermal growth factor, transforming growth factor alpha and their receptor in human prostate tissue and cell lines. Molec Cell Biochem, 126, 151–8.
Tillotson JK and Rose DP, 1991, Endogenous secretion of epidermal growth factor peptides stimulates growth of DU145 prostate cancer cells. Cancer Lett, 60, 109–12.
Haugen DRF, Akslen LA, Varhaug JE and Lillehaug JR, 1993, Demonstration of a TGF-a-EGF-receptor autocrine loop and c-myc protein over-expression in papillary thyroid carcinomas. Int J Cancer, 55, 37–43.
Hamada J, Nagayasu H, Takayama M, Kawano T, Hosokawa M and Takeichi N, 1995, Enhanced effect of epidermal growth factor on pulmonary metastasis and in vitro invasion of rat mammary carcinoma cells. Cancer Lett, 89, 161–7.
Chakrabarty S, Rajagopal S and Huang S, 1995, Expression of antisense epidermal growth factor receptor RNA downmodulates the malignant behavior of human colon cancer cells. Clin Exp Metastasis, 13, 191–5.
Korc M, Chandrasekar B, Yamanaka Y, et al., 1992, Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest, 90, 1352–60.
Stone K, Mickey DD, Wunderli H, Mickey GH and Paulson DF, 1978, Isolation of a human prostate carcinoma cell line (DU145). Int J Cancer, 21, 274–81.
Connolly JM and Rose DP, 1992, Interactions between epidermal growth factor-mediated autocrine regulation and linoleic acid-stimulated growth of a human prostate cancer cell line. Prostate, 20, 151–8.
Xie H, Turner T, Wang M-H, Singh RK, Siegal GP and Wells A, 1995, In vitro invasiveness of DU-145 human prostate carcinoma cells is modulated by EGF receptor-mediated signals. Clin Exp Metastasis, 13, 407–19.
Chen P, Gupta K and Wells A, 1994, Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J Cell Biol, 124, 547–55.
Chen P, Xie H, Sekar MC, Gupta KB and Wells A, 1994, Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but MAP kinase activity is not sufficient for induced cell movement. J Cell Biol, 127, 847–57.
Bleasdale JE, Thakur NR, Gremban RS, et al., 1990, Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharm Exp Ther, 255, 756–68.
Powis G, Seewald MJ, Gratas C, Melder D, Riebow J and Modest EJ, 1992, Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues. Cancer Res, 52, 2835–40.
Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN and Rosenfeld MG, 1990, Ligand-induced transformation by a non-internalizing EGF receptor. Science, 247, 962–4.
Ullrich A, Coussens L, Hayflick JS, et al., 1984, Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature, 307, 418–25.
Welsh JB, Gill GN, Rosenfeld MG and Wells A, 1991, A negative feedback loop attenuates EGF-induced morphological changes. J Cell Biol, 114, 533–43.
Masui H, Wells A, Lazar CS, Rosenfeld MG and Gill GN, 1991, Enhanced tumorigenesis of NR6 cells which express non-downregulating epidermal growth factor receptors. Cancer Res, 51, 6170–5.
Service NTI, 1981–82, Registry of Toxic Effects of Chemical Substance. Washington DC: US Department of Commerce.
Peterson G and Barnes S, 1993, Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate, 22, 335–45.
Knox JD, Mack CF, Powell WC, Bowden GT and Nagle RB, 1993, Prostate tumor cell invasion: a comparison of orthotopic and ectopic models. Invasion and Metastasis, 13, 325–31.
Powell WC, Knox JD, Navre M, et al., 1993, Expression of the metalloproteinase matrilysin in DU145 cells increases their invasive potential in severe combined immunodeficient mice. Cancer Res, 53, 417–22.
Sunada H, Magun BE, Mendelsohn J and MacLeod CL, 1986, Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proc Natl Acad Sci, USA, 83, 3825–9.
Chen P, Xie H and Wells A, 1996, Mitogenic signaling from the EGF receptor is attenuated by a motility-associated phospholipase C-γ/protein kinase C feedback mechanism. Molec Biol Cell, 7, in press.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turnert, T., Chen, P., Goodly, L.J. et al. EGF receptor signaling enhances in vivo invasiveness of DU-145 human prostate carcinoma cells. Clin Exp Metast 14, 409–418 (1996). https://doi.org/10.1007/BF00123400
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00123400