Summary
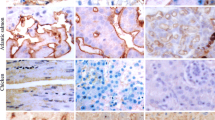
The choriocapillaris is one example of a capillary bed lined by a fenestrated endothelium that is restrictive to exogenous tracers and endogenous plasma proteins. In this study we have examined the distribution of cell-surface monosaccharides utilizing biotinylated lectin-avidin ferritin cytochemistry. Receptors for wheat germ agglutinin were localized to the plasmalemma and diaphragms of some fenestrae, vesicles, and channels at the luminal endothelial front in amounts greater than seen for the other lectins employed. The absence of labeling following inhibition with N-acetylglucosamine and after tissue digestion with N-acetylhexosaminidase, but not after neuraminidase indicated that this lectin marked N-acetylglucosamine residues and not sialic acid. Wheat germ agglutinin receptors were not affected by pronase E or trypsin digestion, but were partially removed by proteinase K. The latter also removed many fenestral diaphragms. Wheat germ agglutinin receptors were cleaved with endoglycosidase D. The combined results indicate that the wheat germ agglutinin receptor is of the low-mannose type and part of a protein with hydrophobic properties. Receptors for concanavalin A (mannose) and Ricinus communis agglutinin (galactose) were also localized to the plasmalemma and endothelial diaphragms. The examination of sections at different tilt angles revealed that these lectins bound to the endothelium in a non-random distribution, encircling diaphragms of fenestrae and channels. Soybean agglutinin (N-acetylgalactosamine) marked endothelial structures sparsely. Following digestion with pronase E or trypsin, receptor sugars for the latter three lectins were completely removed, indicating their presence on protease susceptible glycoproteins. These findings demonstrate that the endothelium of the choriocapillaris bears carbohydrate moieties that are different than those described for permeable fenestrated endothelia.
Similar content being viewed by others
References
Bankston PW, Milici AJ (1983) A survey of the binding of polycationic ferritin in several fenestrated capillary beds: indication of heterogeneity in the luminal glycocalyx of fenestral diaphragms. Microvasc Res 26:36–48
Bearer EL, Orci L (1985) Endothelial fenestral diaphragms: a quick-freeze, deep-etch study. J Cell Biol 100:418–428
Bignon J, Jaubert F, Jaurand MC (1976) Plasma protein immunocytochemistry and polysaccharide cytochemistry at the surface of alveolar and endothelial cells in the rat lung. J Histochem Cytochem 24:1076–1084
Bretton R, Bariety J (1974) Ultrastructural localization of concanavalin A in normal rat kidney glomeruli and arterioles. J Ultrastruct Res 48:396–403
Bretton R, Bariety J (1976) A comparative ultrastructural localization of concanavalin A, wheat germ agglutinin and Ricinus communis on glomeruli of normal rat kidney. J Histochem Cytochem 24:1093–1100
Clementi F, Palade GE (1969a) Intestinal capillaries. I. Permeability to peroxidase and ferritin. J Cell Biol 41:33–58
Clementi F, Palade GE (1969b) Intestinal capillaries. II. Structural effects of EDTA and histamine. J Cell Biol 42:706–714
Debray H, Decout D, Strecker G, Spik G, Montreuil J (1981) Specificity of twelve lectins towards oligosaccharides and glyco-peptides related to N-glycosylproteins. Eur J Biochem 117:41–55
Ebling W, Hennrich N, Klockow M, Metz H, Orth HD, Lang H (1974) Proteinase K from Tritirachium album limber. Eur J Biochem 47:91–97
Essner E, Gordon SR (1983) Observations on the permeability of the choriocapillaris of the eye. Cell Tissue Res 231:571–577
Goldstein U, Hayes CE (1978) The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem 35:127–340
Gordon JA, Staehelin LA, Kuettner CA (1977) Lectin-mediated agglutination of erythrocyte ghost membranes following depletion of membrane protein and intramembrane particles. Exp Cell Res 110:439–448
Graham RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney. Ultrastructural cytochemistry by a new technique. J Histochem Cytochem 15:291–302
Green NM (1963) Avidin. I. The use of 14C biotin for kinetic studies and for assay. Biochem J 89:585–591
Holthofer H, Virtnen I, Kariniemi A-L, Hormia M, Linder E, Miettinen A (1982) Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest 47:60–66
Hurley JV, McCallum NEW (1974) The degree and functional significance of the escape of marker particles from small blood vessels with fenestrated endothelium. J Pathol 113:183–196
Koide N, Muramatsu T (1974) End-β-N-acetylglucosaminidase acting on carbohydrate moieties of glycoproteins. Purification and properties of the enzymes from Diplococcus pneumoniae. J Biol Chem 249:4897–4904
Kornfeld R, Kornfeld S (1970) The structure of a phytohemagglutinin receptor from human erythrocytes. J Biol Chem 245:2536–2545
Linker A, Hovingh P (1972) Heparinase and heparitinase from Flavobacteria. In Ginsberg V (ed) Methods in enzymology, Vol 28. Academic Press, New York, pp 902–911
Luft JH (1961) Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol 9:409–414
Monsigny M, Roche A-C, Sene C, Maget-Dana R, Delmotte F (1980) Sugar-lectin interactions: how does wheat-germ agglutinin bind sialoglycoconjugates? Eur J Biochem 104:147–153
Morihara K, Tsuzuki H (1975) Specificity of proteinase K from Tritirachium album limber for synthetic peptides. Agr Biol Chem 39:1489–1492
Muller LI, Jacks TJ (1975) Rapid chemical dehydration of samples for electron microscopic examinations. J Histochem Cytochem 23:107–110
Nicolson GL (1976) Trans-membrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over cell surface components. Biochim Biophys Acta 457:57–108
Novikoff AB, Novikoff PM, Davis C, Quintana N (1972) Diffusion artifacts in 3,3′-diaminobenzidine cytochemistry. J Histochem Cytochem 20:745–749
Peters K-R, Milici AJ (1983) High resolution scanning electron microscopy of the luminal surface of a fenestrated capillary endothelium. J Cell Biol 97: 336a
Pino RM (1984a) Ultrastructural localization of lectin receptors on the bone-marrow sinusoidal endothelium of the rat. Am J Anat 169:259–272
Pino RM (1984b) Ultrastructural localization of lectin receptors on the surface of the rat retinal pigment epithelium. Decreased sensitivity of the avidin-biotin method due to cell surface charge. J Histochem Cytochem 32:862–868
Pino RM (1984c) Heparin and heparan sulfate domains on a fenestrated endothelium. Third Intl Symposium on the Biology of the Vascular Endothelial Cell, Cambridge, MA, abstracts: 32
Pino RM (1985) Restriction to endogenous plasma proteins by a fenestrated capillary endothelium: an ultrastructural immunocytochemical study of the choriocapillary endothelium. Am J Anat 172:279–289
Pino RM (1986) The cell surface of a restrictive fenestrated endothelium. II. Dynamics of cationic ferritin binding and the identification of heparin and heparan sulfate domains on the choriocapillaris. Cell Tissue Res 243:157–164
Pino RM, Essner E (1980) Structure and permeability to ferritin of the choriocapillary endothelium of the rat eye. Cell Tissue Res 208:21–27
Pino RM, Essner E (1981) Permeability of rat choriocapillaris to hemeproteins. Restriction of tracers by a fenestrated endothelium. J Histochem Cytochem 29:281–290
Pino RM, Thouron CL (1983) Vascular permeability in the rat eye to endogenous albumin and immunoglobulin G (IgG) examined by immunohistochemical methods. J Histochem Cytochem 31:411–416
Pino RM, Essner E, Pino LC (1982a) Permeability of the neonate rat choriocapillaris to hemeproteins and ferritin. Am J Anat 164:333–341
Pino RM, Essner E, Pino LC (1982b) Location and chemical composition of anionic sites in Bruch's membrane of the rat. J Histochem Cytochem 30:245–252
Rafelson ME, Gold S, Priede I (1966) Neuraminidase (sialidase) from Influenza virus. In Neufeld EF, Ginsberg V (eds) Methods in enzymology, Vol 8. Academic Press, New York, pp 677–680
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 67:638–646
Schneeberger EE, Hamelin M (1984) Interaction of serum proteins with lung endothelial glycocalyx: its effect on endothelial permeability. Am J Physiol 247 (Heart Circ Physiol 16):H206-H217
Sharon N, Lis H (1982) Glycoproteins. In Neurath H, Hill RL (eds) The proteins, Vol 5. Academic Press, New York, pp 1–144
Shimamura T, Morrison AB (1973) Vascular permeability of the medullary vessels in the mouse and rat. Am J Pathol 71:155–166
Simionescu M, Simionescu N, Silbert JE, Palade GE (1981) Differentiated microdomains on the luminal surface of the capillary endothelium. II. Partial characterization of their anionic sites. J Cell Biol 90:614–621
Simionescu M, Simionescu N, Palade GE (1982) Differentiated microdomains on the luminal surface of capillary endothelium: Distribution of lectin receptors. J Cell Biol 71:218–231
Simionescu N, Simionescu M, Palade GE (1972) Permeability of intestinal capillaries. Pathway followed by dextrans and glycogens. J Cell Biol 53:365–392
Simionescu N, Lupu F, Simionescu M (1983) Rings of membrane sterols surround the openings of vesicles and fenestrae, in capillary endothelium. J Cell Biol 97:1592–1600
Stein O, Chajek T, Stein Y (1976) Ultrastructural localization of concanavalin A in the perfused rat heart. Lab Invest 35:103–110
Venkatachalam MA, Karnovsky MJ (1972) Extravascular protein in the kidney. An ultrastructural study of its relation to renal peritubular capillarypermeability using protein tracers. Lab Invest 27:435–444
Weber G, Fabbrini P, Resi L (1973) On the presence of concanavalin A reactive coat over the endothelial aortic surface and its modification during early experimental cholesterol atherogenesis in rabbits. Virchows Arch (Pathol Anat) 359:299–307
Wolff JR (1977) Ultrastructure of the terminal vascular bed as related to function. In: Kaley G, Altura BM (eds) Microcirculation. University Park Press, Baltimore, pp 95–130
Yamamoto K, Tsutomu T, Matsumoto I, Osawa T (1981) Structural requirements for the binding of oligosaccharides and glycopeptides to immobilized wheat germ agglutinin. Biochemistry 20:5894–5899
Author information
Authors and Affiliations
Additional information
Supported by NIH EY 03776
Rights and permissions
About this article
Cite this article
Pino, R.M. The cell surface of a restrictive fenestrated endothelium. Cell Tissue Res. 243, 145–155 (1986). https://doi.org/10.1007/BF00221863
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00221863