Summary
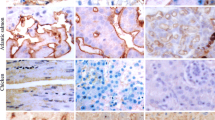
The location and chemical composition of anionic sites on the endothelium of the choriocapillaris was investigated with cationic ferritin and enzyme digestion techniques. Cationic ferritin administered intravenously initially labeled essentially all fenestral diaphragms. Within 30 min after injection, no diaphrams remained labeled, but they could be relabeled by a second cationic ferritin injection. Following perfusion of cationic ferritin, the entire luminal front of the endothelium was labeled: the plasmalemma and fenestral, vesicle, and channel diaphragms. Perfusion of neuraminidase or chondroitinase did not affect subsequent cationic ferritin binding. In contrast, heparitinase removed anionic sites on all structures except fenestral diaphragms. Cationic ferritin did not mark the endothelium following heparinase digestion. All sites were cleaved with pronase E. These results indicate that heparin is the anionic moiety on fenestral diaphragms while the glycocalcyces of the plasmalemma and vesicle and channel diaphragms are rich in a heparan sulfate proteoglycan. Furthermore, since the heparan sulfate localized to these structures was digested by both heparinase and heparitinase, it is in a form similar to heparin. These findings demonstrate that the endothelium of the choriocapillaris bears cell-surface anionic components that are different than those described for fenestrated endothelia lining other vascular beds.
Similar content being viewed by others
References
Bankston PW, Milici AJ (1983) A survey of the binding of polycationic ferritin in several fenestrated capillary beds: indication of heterogeneity in the luminal glycocalyx of fenestral diaphragms. Microvasc Res 26:36–48
Castellot JJ, Wong K, Herman B, Albertini DF, Wright TC, Hoover RL, Karnovsky MJ (1983) Binding and internalization of heparin by vascular smooth muscle cells. J Cell Biol 97:430a
Danon D, Goldstein L, Marikovsky Y, Skutelsky E (1972) Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res 38:500–510
De Bruyn PPH, Michelson S (1979) Changes in the random distribution of sialic acid at the surface of the myeloid sinusoidal endothelium resulting from the presence of diaphragmed fenestrae. J Cell Biol 82:708–714
Dermietzel R, Thurauf N, Kaiweit P (1983) Surface charges associated with fenestrated brain capillaries. II. In vivo studies on the role of molecular charge in endothelial permeability. J Ultrastruct Res 84:111–119
Jaques LB (1977) Determination of heparin and related sulfated mucopolysaccharides. Methods Biochem Anal 24:201–312
Kanwar Y, Farquhar MG (1979) Anionic sites in the glomerular basement membrane. J Cell Biol 81:137–153
Linker A, Hovingh P (1972) Heparinase and heparitinase from Flavobacteria. In: Ginsberg V (ed) Methods in enzymology, Vol 28. Academic Press, New York, pp 902–911
Luft JH (1961) Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol 9:409–414
Mandl I, MacLennan JD, Howes EL (1953) Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J Clin Invest 32:1323–1329
Muller LI, Jacks TJ (1975) Rapid chemical dehydration of samples for electron microscopic examinations. J Histochem Cytochem 23:107–110
Pino RM (1984) Heparin and heparan sulfate domains on a fenestrated endothelium. Third Intl Symposium on the Biology of the Vascular Endothelial Cell, Cambridge, MA, abstracts:32
Pino RM (1985a) Restriction to endogenous plasma proteins by a fenestrated capillary endothelium: an ultrastructural immunocytochemical study of the choriocapillary endothelium. Am J Anat 172:279–289
Pino RM (1985b) Heparin and the rat choriocapillary endothelium. Invest Ophthal Vis Sci 26, Suppl: 327
Pino RM (1986) The cell surface of a restrictive fenestrated endothelium. I. Distribution of lectin-receptor monosaccharides on the choriocapillaris. Cell Tissue Res 243:145–155
Pino RM, Essner E (1980) Structure and permeability to ferritin of the choriocapillary endothelium of the rat eye. Cell Tissue Res 208:21–27
Pino RM, Essner E (1981) Permeability of rat choriocapillaris to hemeproteins. Restriction of tracers by a fenestrated endothelium. J Histochem Cytochem 29:281–290
Pino RM, Hart TK (1984) Isoelectric focusing in polyacrylamideagarose. Anal Biochem 139:77–81
Pino RM, Essner E, Pino LC (1982) Location and chemical composition of anionic sites in Bruch's membrane of the rat. J Histochem Cytochem 30:245–252
Rafelson ME, Gold S, Priede I (1966) Neuraminidase (sialidase) from Influenza virus. In: Neufeld EF, Ginsberg V (eds) Methods in enzymology, Vol. 8. Academic Press, New York, pp 677–680
Rennke HG, Cotran RS, Venkatachalam MA (1975) Role of molecular charge in glomerular permeability. J Cell Biol 67:638–646
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:155–159
Schneeberger EE, Hamelin M (1984) Interaction of serum proteins with lung endothelial glycocalyx: its effect on endothelial permeability. Am J Physiol 247 (Heart Circ Physiol 16):H206-H217
Simionescu M, Simionescu N, Silbert JE, Palade GE (1981 a) Differentiated microdomains on the luminal surface of the capillary endothelium. II. Partial characterization of their anionic sites. J Cell Biol 90:614–621
Simionescu N, Simionescu M, Palade GE (1981b) Differentiated microdomains on the luminal surface of the capillary endothelium. I. Preferential distribution of anionic sites. J Cell Biol 90:605–613
Skutelsky E, Danon D (1976) Redistribution of surface anionic sites on the luminal front of blood vessel endothelium after interaction with polycationic ligand. J Cell Biol 71:232–241
Thurauf N, Dermietzel R, Kaiweit P (1983) Surface charges associated with fenestrated brain capillaries. I. In vitro labeling of anionic sites. J Ultrastruct Res 84:103–110
Author information
Authors and Affiliations
Additional information
Supported by NIH EY 03776
Rights and permissions
About this article
Cite this article
Pino, R.M. The cell surface of a restrictive fenestrated endothelium. Cell Tissue Res. 243, 157–164 (1986). https://doi.org/10.1007/BF00221864
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00221864