Abstract
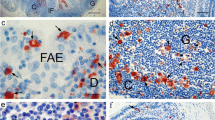
The follicle-associated epithelium (FAE) in the rabbit caecal lymphoid patch is characterised by the presence of membranous (M) cells, which are believed to be functionally equivalent to those present at other sites of gut-associated lymphoid tissue (GALT). Caecal patch M cells display distinctive features compared with those of other GALT sites, despite similar general morphology and expression of the M cell marker vimentin, suggesting marked heterogeneity in the apical surface of M cells at discrete GALT sites. Electron microscopy reveals that rabbit caecal patch M cells differ from those in the small intestinal Peyer's patch FAE: the former have a prominent aspect within the epithelium and possess microvilli which are longer than those of adjacent enterocytes. Many of the M cells in peripheral regions of the caecal patch FAE are not associated with leucocytes and may thus represent an immature M cell population. The M cells are also histochemically distinct from adjacent enterocytes and from Peyer's patch M cells, showing greater expression of brush-border alkaline phosphatase activity and affinity for certain lectins (peanut and wheat germ agglutinins, Bandeiraea simplicifolia agglutinin II). The differences in the brush-border morphology and glycocalyx structure between M cells at different GALT sites may affect their function at these sites by influencing the interaction of luminal antigens and microorganisms with the M cell surface. The present data also support the hypothesis that M cells arise directly from differentiation of crypt stem cells and not from the transformation of existing fully differentiated enterocytes.
Similar content being viewed by others
References
Bhalla DK, Owen RL (1982) Cell renewal and migration in lymphoid follicles of Peyer's patches and cecum — An autoradiographic study in mice. Gastroenterology 82:232–242
Bjerke K, Brandtzaeg P (1988) Lack of relation between expression of HLA-DR and secretory component (SC) in follicle-associated epithelium of human Peyer's patches. Clin Exp Immunol Immunopathol 71:502–507
Bockman DE, Cooper MD (1973) Pinocytosis by the epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix and Peyer's patches. An electron microscopical study. Am J Anat 136:455–478
Bye WA, Allan CH, Trier JS (1984) Structure, distribution and origin of M cells in Peyer's patches of mouse ileum. Gastroenterology 86:789–801
Clark MA, Jepson MA, Simmons NL, Booth TA, Hirst BH (1993) Differential expression of lectin binding sites defines mouse intestinal M cells. J Histochem Cytochem 41:1679–1687
Finzi G, Cornaggia M, Capella C, Fiocca R, Bosi F, Solcia E, Samloff IM (1993) Cathepsin E in follicle associated epithelium of intestine and tonsils: localization to M cells and possible role in antigen processing. Histochemistry 99:201–211
Gebert A, Bartels H (1991) Occluding junctions in the epithelia of the gut-associated lymphoid tissue (GALT) of the rabbit ileum and caecum. Cell Tissue Res 266:301–314
Gebert A, Hach G, Bartels H (1992) Co-localization of vimentin and cytokeratins in M-cells of rabbit gut-associated lymphoid tissue (GALT). Cell Tissue Res 269:331–340
Hogenesch H, Felsburg PJ (1990) Ultrastructure and alkaline phosphatase activity of the dome epithelium of canine Peyer's patches. Vet Immunol Immunopathol 24:177–186
Jepson MA, Mason CM, Bennett MK, Simmons NL, Hirst BH (1992a) Co-expression of vimentin and cytokeratins in M cells of rabbit intestinal lymphoid follicle-associated epithelium. Histochem J 24:33–39
Jepson MA, Simmons NL, Hirst BH (1992b) Heterogeneity of epithelia in rabbit gut-associated lymphoid tissues. J Physiol 452:359P
Jepson MA, Simmons NL, Hirst GL, Hirst BH (1993a) Identification of M cells and their distribution in rabbit intestinal Peyer's patches and appendix. Cell Tissue Res 273:127–136
Jepson MA, Simmons NL, Savidge TC, James PS, Hirst BH (1993b) Selective binding and transcytosis of latex microspheres by rabbit intestinal M cells. Cell Tissue Res 271:399–405
Jepson MA, Simmons NL, O'Hagan DT, Hirst BH (1993c) Comparison of poly(dl-lactide-co-glycolide) and polystyrene microsphere targeting to intestinal M cells. J Drug Targeting 1:245–249
Kato T (1990) A study of secretory immunoglobulin A on membranous epithelial cells (M cells) and adjacent absorptive cells of rabbit Peyer's patches. Gastroenterol Jpn 25:15–23
Kraehenbuhl J-P, Neutra JP (1992) Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev 72:853–879
Neutra MR, Phillips TL, Mayer EL, Fishkind DJ (1987) Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer's patch. Cell Tissue Res 247:537–546
Owen RL, Bhalla DK (1983) Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse Peyer's patch M cells. Am J Anat 168:199–212
Owen RL, Nemanic P (1978) Antigen processing structures of the mammalian intestinal tract: an SEM study of lymphoepithelial organs. Scan Electron Microsc 11:367–378
Owen RL, Cray WC, Ermak TH, Pierce NF (1988) Bacterial characteristics and follicle surface structure: their roles in Peyer's patch uptake and transport of Vibrio cholerae. Adv Exp Med Biol 237:705–715
Pappo J (1989) Generation and characterization of monoclonal antibodies recognizing follicle epithelial M cells in rabbit gut-associated lymphoid tissues. Cell Immunol 120:31–41
Roy MJ, Ruiz A, Varvayanis M (1987) A novel antigen is common to the dome epithelium of gut- and bronchus-associated lymphoid tissues. Cell Tissue Res 248:635–644
Smith MW, Peacock MA (1980) “M” cell distribution in follicle-associated epithelium of mouse Peyer's patch. Am J Anat 159:167–175
Smith MW, Peacock MA (1992) Microvillus growth and M-cell formation in mouse Peyer's patch follicle-associated epithelial tissue. Exp Physiol 77:389–392
Smith MW, James PS, Tivey DR, Brown D (1988) Automated histochemical analysis of cell populations in the intact follicle-associated epihelium of the mouse Peyer's patch. Histochem J 20:443–448
Snipes RL (1978) Anatomy of the rabbit cecum. Anat Embryol 155:57–80
Trier JS (1991) Structure and function of intestinal M cells. Gastroenterol Clin N Am 20:531–547
Uchida J (1987) An ultrastructural study on active uptake and transport of bacteria by microfold cells (M cells) to the lymphoid follicles in the rabbit appendix. J Clin Electron Microsc 20:379–394
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jepson, M.A., Clark, M.A., Simmons, N.L. et al. Epithelial M cells in the rabbit caecal lymphoid patch display distinctive surface characteristics. Histochemistry 100, 441–447 (1993). https://doi.org/10.1007/BF00267824
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00267824