Summary
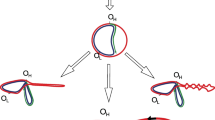
In a random collection of mit− mutations of the yeast strain 777-3A we find that deletions are exceptionally frequent in the OXI3 gene, a large mosaic gene coding for subunit I of cytochrome oxidase. About 10% of all oxi3 − mutants carry the same macro-deletion, del-A, extending from the 5′ non-translated leader of OXI3 to intron 5b of this gene. Determination of the respective wild-type sequences and of the del-A junction sequence revealed that the end-points of the deletion are in two GC clusters with 31 by sequence identity which are located at a distance of 11.3 kb. We speculate that not only the sequence identity of the two GC clusters but also the palindromic structure of these putatively mobile elements of yeast mitochondrial DNA (mtDNA) plays a role in deletion formation.
Similar content being viewed by others
References
Ahne A, Müller-Derlich J, Merlos-Lange AM, Kanbay F, Wolf K, Lang BF (1988) Two distinct mechanisms of deletion in mitochondrial DNA of Schizosaccharomyces pombe mutation strains: Slipped mispairing mediated by direct repeats and erroneous intron splicing. J Mol Biol 202:725–734
Bechmann H, Haid A, Schweyen RJ, Mathews S, Kaudewitz F (1981) Expression of the split gene COB in yeast mtDNA: Translation of intervening sequences in mutant strains. J Biol Chem 256:3525–3531
Bonitz SG, Coruzzi G, Thalenfeld BE, Tzagoloff A, Macino G (1980) Assembly of the mitochondrial membrane system: Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrome oxidase. J Biol Chem 255:11927–11941
Butow RA, Perlman PS, Grossman LI (1985) The unusual VAR1 gene of yeast mitochondrial DNA. Science 228:1496–1501
Carignani G, Dujardin G, Slonimski PP (1979) Petite deletion map of the mitochondrial oxi3 region in Saccharomyces cerevisiae. Mol Gen Genet 167:301–308
Clark-Walker GD (1989) in vivo rearrangements of mitochondrial DNA in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 86:8847–8851
Coruzzi G, Tremblath MK, Tzagoloff A (1978) Assembly of the mitochondrial membrane system: Mutations in the pho2 locus of the mitochondrial genome of Saccharomyces cerevisiae. Eur J Biochem 92:279–287
De Zamaroczy M, Bernardi G (1986) The GC clusters of the mitochondrial genome of yeast and their evolutionary origin. Gene 41:1–22
De Zamoroczy M, Faugeron-Fonty G, Bernardi G (1983) Excision sequences in the mitochondrial genome of yeast. Gene 21:193–202
Dieckmann CL, Gandy B (1987) Preferential recombination between GC clusters in yeast mitochondrial DNA. EMBO J 6:4197–4203
Dobres M, Gerbl-Rieger S, Schmelzer C, Mueller MW, Schweyen RJ (1985) Deletions in the cob gene of yeast mRNA and their phenotypic effect. Curr Genet 10:283–290
Dujon B, Belcour L (1989) Mitochondrial DNA instabilities and rearrangements in yeast and fungi. In: Berg DE, Howe MH (eds) Mobile DNA. Washington DC, pp 861–878
Ehrlich SD (1989) Illegitimate recombination in bacteria. In: Berg DE, Howe MH (eds) Mobile DNA. American Society of Microbiology. Washingon DC, pp 799–832
Franklin NC (1971) Illegitimate recombination. In: Hershey AD (ed) The bacteriophage lambda. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 175–194
Gaillard C, Strauss F, Bernardi G (1980) Excision sequences in the mitochondrial genome of yeast. Nature 283:218–220
Gough J, Murray NE (1983) Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol 166:1–19
Grivell LA (1989) Small, beautiful and essential. Nature 341:569–571
Hardy CM, Galeotti CL, Clark-Walker GD (1990) Deletions and rearrangements in Kluyveromyces lactis mitochondrial DNA. Curr Genet 10:419–427
Hensgens LAM, Bonen L, De Haan M, Van Der Horst G, Grivell LA (1983) Two introns sequences in yeast mitochondrial COX1 gene: Homology among urf-containing introns and strain-dependent variation in flanking exons. Cell 32:379–389
Hensgens LAM, Van Der Horst G, Vos HL, Grivell LA (1984) RNA processing in yeast mitochondria: characterization of mit− mutants disturbed in synthesis of subunit I of cytochrome oxidase. Curr Genet 8:457–465
Kotylak S, Slonimski PP (1977) Mitochondrial mutants isolated by a new screening method based upon the use of the nuclear mutation op1. In: Bandlow W, Schweyen RJ, Wolf K, Kaudewitz F (eds) Mitochondria 1977: Genetics and biogenesis of mitochondria. de Gruyter, Berlin, pp 83–89
Kruszewska A, Szczesniak B, Claisse M (1980) Recombinational analysis of OXI1 mutants and preliminary analysis of their translation products in S. cerevisiae. Curr Genet 2:45–51
Lancashire WE, Mattoon JR (1979) Cytoduction: A tool for mitochondrial genetics in yeast. Mol Gen Genet 146:117–132
Levings III CS, Brown GG (1989) Molecular biology of plant mitochondria. Cell 56:171–179
Maxam AM, Gilbert W (1980) A new method for sequencing DNA. Methods Enzymol 65:499–560
Messing J, Crea R, Seeburg PH (1981) A system for shotgun DNA sequencing. Nucl Acids Res 9:309–321
Messing J (1983) New M13 vectors for cloning. Methods Enzymol 101:20–78
Meuth M (1989) Illegitimate recombination in mammalin cells. In: Berg DE, Howe MH (eds) Mobile DNA. Washington, DC pp 833–860
Myers AM, Pape LK, Tzagoloff A (1985) Mitochondrial protein synsthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J 4:2087–2092
Osinga KA, Vries EDE, Van der Horst GTJ, Tabak HF (1984) Initiation of transcription in yeast mitochondria: Analysis of origin of replication and of genes coding for a messenger RNA and a transfer RNA. Nuclei Acids Res 12:1889–1900
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schon EA, Rizzuto R, Moraes CT, Nakase H, Zeviani M, DiMauro D (1989) A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science 244:346–349
Schweyen RJ, Steyrer U, Kaudewitz F, Dujon F, Slonimski PP (1976) Mapping of mitochondrial genes in Saccharomyces cerevisiae. Population and pedigree analysis of retention or loss of four genetic markers in rho− cells. Mol Gen Genet 146:117–132
Schweyen RJ, Weiss-Brummer B, Backhaus B, Kaudewitz F (1978) The genetic map of the mitochondrial genome in yeast. Mol Gen Genet 159:151–160
Slonimski PP, Tzagoloff A (1976) Localization in yeast mitochondrial DNA of mutations expressed in a deficiency of cytochrome oxidase and/or coenzyme QH2-cytochrome c reductase. Eur J Biochem 61:27–41
Wallace DC (1989) Mitochondrial DNA mutations and neuromuscular disease. Trends Genet 5:9–13
Weiller G (1987) Mobile Elemente im mitochondrialen Genom der Hefe. PhD Thesis, University of Munich
Weiller G, Schueller CME, Schweyen RJ (1989) Putative target sites for mobile G+C rich clusters in yeast mitochondrial DNA: Single elements and tandem arrays. Mol Gen Genet 218:272–283
Weiss-Brummer B, Guba R, Haid A, Schweyen RJ (1979) Fine structure of OXI1, the mitochondrial gene coding for subunit II of yeast cytochrome c oxidase. Curr Genet 1:75–83
Zinn AR, Pohlmann JK, Perlman PS, Butow RA (1988) In vivo double stand breaks at recombinogenic G+C rich sequences in the yeast mitochondrial genome. Proc Natl Acad Sci USA 85:2686–2690
Author information
Authors and Affiliations
Additional information
Communicated by W. Gajewski
Rights and permissions
About this article
Cite this article
Weiller, G.F., Bruckner, H., Kim, S.H. et al. A GC cluster repeat is a hotspot for mit− macro-deletions in yeast mitochondrial DNA. Mol Gen Genet 226, 233–240 (1991). https://doi.org/10.1007/BF00273608
Issue Date:
DOI: https://doi.org/10.1007/BF00273608