Abstract
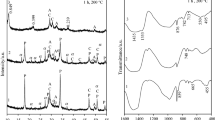
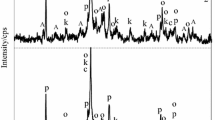
Mixtures of CaO, amorphous SiO2 and kaolinite in the presence or absence of SO 2−4 ions (added as CaSO4·2H2O) were treated in suspension under hydrothermal conditions at temperatures of 80–200 °C. Kaolinite was added to replace 3 and 10% of the total weight of the dry mix with the overall CaO/SiO2 mole ratio being 0.83. The hydration products were investigated by XRD, IR spectroscopy and DTA techniques in order to elucidate their phase compositions. The results indicate that the presence of SO 2−4 ions leads to a marked increase in the reaction rate at all temperatures investigated. In the C-S-A system, the detected hydration products are calcium silicate hydrate which is then transformed into 1.13 nm aluminium-substituted tobermorite and α-C2SH by increasing the autoclaving temperature. In the C-S-A-C¯s system the hydrated products are calcium silicate hydrate, ettringite and monosulpho-alumirtate. On increasing the hydrothermal temperature they decompose, recrystallize and 1.13 nm aluminium-substituted tobermorite, α-C2SH and anhydrite II are formed. In both systems the excess Al2O3 appeared as a hydrogarnet phase, C3ASH4.
Similar content being viewed by others
Abbreviations
- C:
-
CaO
- S:
-
SiO2
- A:
-
Al2O3
- H:
-
H2O
- CH:
-
Ca(OH)2
- C¯s:
-
CaSO4
References
G. L. Kalousek, J. Amer. Ceram. Soc. 40 (1967) 74.
S. Diamond, J. L. White and W. L. Dolch. Amer. Mineral. 51 (1966) 388.
J. Petrovic, Chem. Zresti (Slovak.) 23 (1969) 50.
S. A. S. El-Hemaly, PhD thesis, Aberdeen University, UK (1975).
M. Sakiyama and T. Mitsuda, Cement Concr. Res. 7 (1977) 681.
L. E. Copland, E. Boder, T. N. Chang and C. H. Wise, J. PCA Res. Dev. Lab. 9 (1967) 61.
K. Takemoto and H. Kato, in The 5th International Symposium on the Chemistry of Cement, Tokyo, 1968, (Cement Assoc. of Japan) III-80.
E. I. Al-Wakeel, PhD thesis, TH-Aachen, Germany (1988).
A. J. Easton, “Chemical Analysis of Silicate Rocks” (Elsevier Publ. Co., Amsterdam/London/New York, 1972) p. 93.
J. A. Gadsden, “Infrared Spectra of Minerals and Related Inorganic Compounds” (Butterworth and Co (Publishers) Ltd, London, 1975).
S. A. El-Korashy, PhD thesis. Suez Canal University, Ismailia, Egypt (1991).
S. P. Mukherzee and S. K. Shurma, J. Amer. Ceram. Soc. 69 (1986) 806.
N. P. Bansal, ibid.— 71 (1988) 666.
A. A. Khalil, Cement. Concr. Res. 12 (1982) 21.
A. N. Lazarev, “Vibrational Spectra and Structure of Silicate” (c/b Consultant Burea, New York, London, 1972).
J. Ambroise, M. Mura and J. Pera, Cement Concr. Res. 15 (1985) 83.
P. K. Metha and W. Shanba, ibid.— 12 (1982) 121.
V. S. Ramachandran, “Applications of Differential Thermal Analysis in Cement Chemistry” (Chemical Publishing Company, Inc. New York, 1969).
W. Rechen and S. Sprung, Cement Concr. Res. 13 (1983) 119.
H. F. W. Taylor, “The Chemistry of Cements”, Vol. I (Academic Press, London and New York, 1964) 181.
T. Mistsude, Mineral. J. 6 (1970) 143.
Z. Sauman, in 3rd International Symposium on Autoclaved CS building Product, Utrecht, Netherlands (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Al-Wakeel, E.I., El-Korashy, S.A. Reaction mechanism of the hydrothermally treated CaO-SiO2-Al2O3 and CaO-SiO2-Al2O3-CaSO4 systems. Journal of Materials Science 31, 1909–1913 (1996). https://doi.org/10.1007/BF00372207
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00372207