Summary
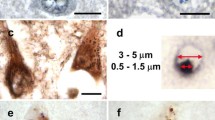
The cationic dyes ruthenium red (RR) and cuprolinic blue (CB) were used to preserve proteoglycans (PGs) for visualization at the ultrastructural level in brain tissue from seven cases of Alzheimer's disease (obtained at autopsy within 3–4 h after death). PGs were visualized as RR-positive granules specifically localized to the amyloid fibrils in neuritic plaques. In neurofibrillary tangles, RR granules were localized to the paired helical filaments and straight filaments usually at a consistent periodicity of 40–70 nm. CB, known to preserve PGs as short punctate filaments, also demonstrated PGs specifically localized to the amyloid fibrils in neuritic plaques and in association with paired helical filaments and straight filaments in neurofibrillary tangles. Persistent staining with CB at magnesium chloride concentrations of 0.3 and 0.7 M in the neuritic plaques suggested the presence of highly sulfated PGs, whereas abolishment of CB staining at 0.7 M magnesium chloride in the neurofibrillary tangles implied that different PGs and/or glycosaminoglycans were present in the neurofibrillary tangles. The specific ultrastructural localization of PGs to the characteristic lesions in Alzheimer's disease suggests that PGs are part of a complex structural network with amyloid fibrils in neuritic plaques and the filamentous structures present in neurofibrillary tangles.
Similar content being viewed by others
References
Abraham CR, Selkoe DJ, Potter H (1988) Immunochemical identification of the serine protease inhibitor alpha-1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell 52:487–501
Benedeczky I, Smith AD (1972) Ruthenium red staining of hamster adrenal medulla. Histochemie 32:213–219
Cole GM, Timiras PS (1987) Ubiquitin-protein conjugates in Alzheimer's lesions. Neurosci Lett 79:207–212
Coria F, Castano E, Prelli F, Larrondo-Lillo M, Van Duinen S, Shelanski ML, Frangione B (1988) Isolation and characterization of amyloid P component from Alzheimer's disease and other types of cerebral amyloidosis. Lab Invest 58:454–458
Delacourte A, Defossez A (1986) Alzheimer's disease: tau proteins, the promoting factors of microtubule assembly, are major components of paired helical filaments. J Neurol Sci 76:173–186
Dorling J (1969) “Critical electrolyte concentration” method in histochemistry. J Med Lab Technol 26:124–130
Gelman RA, Blackwell J (1973) Heparin-polypeptide interactions in aqueous solution. Arch Biochem Biophys 159:427–433
Glenner GG, Wong CW (1984) Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890
Gotlieb L, Bar Sella P, Jaichenko J, Shustack A (1987) Ruthenium red-stained polyanionic fixed charges in peritoneal microvessels. Nephron 47:22–28
Grunke-Iqbal I, Iqbal K, Tung Y, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:4913–4917
Hascall GK (1980) Cartilage proteoglycans: comparison of sectioned and spread whole molecules. J Ultrastruct Res 70:369–375
Holck M, Husby G, Sletten K, Natvig JB (1979) The amyloid P-component (protein AP): an integral part of the amyloid substance? Scand J Immunol 10:55–60
Ihara Y (1986) Phosphorylated tau: a major antigenic determinant of paired helical filaments in Alzheimer's disease. In: Kitani K (ed) Liver and aging. Elsevier Science Publishers, New York, pp 223–231
Iozzo RV (1984) Biosynthesis of heparan sulfate proteoglycans by human colon carcinoma cells and its localization at the cell surface. J Cell Biol 99:403–417
Iozzo RV, Pacifici M (1986) Ultrastructural localization of the major proteoglycan and type II procollagen in organelles and extracellular matrix of cultured chondroblasts. Histochemistry 86:113–122
Iozzo RV, Bolender RP, Wight TN (1982) Proteoglycan changes in the intercellular matrix of human colon carcinoma: an integrated biochemical and morphometric analysis. Lab Invest 47:124–138
Joachim CL, Morris JH, Selkoe DJ, Kosik KS (1987) Tau epitopes are incorporated into a range of lesions in Alzheimer's disease. J Neuropathol Exp Neurol 46:611–622
Kang J, Lemaire H, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K, Multhaup G, Beyreuther K, Muller-Hill B (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325:733–736
Kanwar YS, Farquhar MG (1979) Isolation of glycosaminoglycans (heparan sulfate) from glomerular basement membranes. Proc Natl Acad Sci USA 76:4493–4497
Kirschner DA, Abraham C, Selkoe DJ (1986) X-ray diffraction from intraneuronal paired helical filaments and extraneuronal amyloid fibers in Alzheimer's disease indicates cross-beta conformation. Proc Natl Acad Sci USA 83:503–507
Kosik KS, Joachim CL, Selkoe DJ (1986) Microtubule-associated protein tau is a major antigenic component of paired helical filaments in Alzheimer's disease. Proc Natl Acad Sci USA 83:4044–4048
Luft JH (1971) Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec 171:369–416
Mandybur TI (1986) Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol 45:79–90
Merz PA, Wisniewski HM, Somerville SA, Bobin SA, Masters CL, Iqbal K (1983) Ultrastructural morphology of amyloid fibrils from neuritic and amyloid plaques. Acta Neuropathol (Berl) 60:113–124
Montejo de Garcini E, Serrano L, Avila J (1986) Self assembly of microtubule-associated protein tau into filaments resembling those found in Alzheimer's disease. Biochem Biophys Res Commun 141:790–796
Mori H, Kondo J, Ihara Y (1987) Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science 235:1641–1644
Myers DB, Highton TC, Ryans DG (1969) Acid mucopolysaccharides closely associated with collagen fibrils in normal human synovium. J Ultrastruct Res 28:203–213
Pardridge WM, Vinters HV, Yang J, Eisenberg J, Choi TB, Tourtellotte WW, Huebner V, Shively JE (1987) Amyloid angiopathy of Alzheimer's disease: amino acid composition and partial sequence of a 4,200-dalton peptide isolated from cortical microvessels. J Neurochem 49:1394–1401
Perry G, Freidman R, Shaw G, Chau V (1987) Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites in Alzheimer disease brains. Proc Natl Acad Sci USA 84:3033–3036
Perry G, Mulvihill P, Manetto V, Autoilio-Gambetti L, Gambetti P (1987) Immunocytochemical properties of Alzheimer straight filaments. J Neurosci 7:3736–3738
Phil E, Gustafson GT, Falkmer S (1968) Ultrastructural demonstration of cartilage acid glycosaminoglycans. Histochem J 1:26–35
Scott JE (1972) Histochemistry of alcian blue. III. The molecular biological basis of staining by alcian blue 8GX and analogous phthalocyanins. Histochemie 32:191–212
Scott JE (1980) Collagen-proteoglycan interactions. Localization of proteoglycans in tendon by electron microscopy. Biochem J 187:887–891
Scott JE, Dorling J (1965) Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie 5:221–233
Scott JE, Haigh M (1986) Proteoglycan-collagen interactions in intervertebral disc. A chondroitin sulphate proteoglycan associated with collagen fibrils in rabbit annulus fibrosus at the d-e bands. Biosci Rep 6:879–888
Scott JE, Stockwell RA (1967) On the use and abuse of the critical electrolyte concentration approach to the localization of tissue polyanions. J Histochem Cytochem 15:111–113
Shirahama T, Cohen AS (1972) The role of mucopolysaccharides in vesicle architecture and endothelial transport. J Cell Biol 52:198–206
Skinner M, Cohen AS (1976) Aspects of the amyloid P component. In: Wegelius O, Posternack A (eds) Amyloidosis. Academic Press, New York, pp 339–352
Snow AD, Kisilevsky R (1985) Temporal relationship between glycosaminoglycan accumulation and amyloid deposition during experimental amyloidosis. A histochemical study. Lab Invest 53:37–44
Snow AD, Willmer J, Kisilevsky R (1987) Sulfated glycosaminoglycans: a common constituent of all amyloids? Lab Invest 56:120–123
Snow AD, Willmer J, Kisilevsky R (1987) Sulfated glycosaminoglycans in Alzheimer's disease. Hum Pathol 18:506–510
Snow AD, Kisilevsky R, Stephens C, Anastassiades T (1987) Characterization of tissue and plasma glycosaminoglycans during experimental AA amyloidosis and acute inflammation. Qualitative and quantitative analysis. Lab Invest 56:665–675
Snow AD, Willmer J, Kisilevsky R (1987) A close ultrastructural relationship between sulfated proteoglycans and AA amyloid fibrils. Lab Invest 57:687–698
Snow AD, Mar H, Nochlin D, Wight TN (1988) Congo red staining on 1 micron de-plasticized sections for detection of lesions in Alzheimer's disease and related disorders. Alz Dis Assoc Dis 2:235 [abstr]
Snow AD, Mar H, Nochlin D, Kimata K, Kato M, Suzuki S, Hassell J, Wight TN (1988) The presence of heparan sulfate proteoglycans in the neuritic plaques and congophilic angiopathy in Alzheimer's disease. Am J Pathol 133:456–463
Snow AD, Kisilevsky R, Wight TN (1988) Immunolocalization of heparan sulfate proteoglycans to AA amyloid deposition sites in spleen and liver during experimental amyloidosis. In: Isobe I, Araki S, Uchino F, Kito S, Tsuruba E (eds) Amyloid and amyloidosis. Plenum Press, New York, pp 87–93
Snow AD, Nochlin D, Sumi S, Bird TD, Wight TN (1988) Immunolocalization of heparan sulfate proteoglycans to “primitive plaques” and multi-core prion positive plaques in familial dementia. Alz Dis Assoc Dis 2:232 [abstr]
Stone AL (1977) Optimal conformation of heparin and heparan complexes with cationic dyes, amines and protein models. Fed Proc 36:101–106
Tawara A, Varner HH, Hollyfield JG (1988) Proteoglycans in the mouse interphotoreceptor matrix. I. Histochemical studies using cuprolinic blue. Exp Eye Res 46:689–704
Vacarro CA, Brody JS (1979) Ultrastructural localization and characterization of proteoglycans in the pulmonary alveolus. Am Rev Respir Dis 120:901–910
Van Kuppevelt THMSM, Domen JGW, Cremers FPM, Kuyper CMA (1984) Staining of proteoglycans in mouse lung alveoli. I. Ultrastructural localization of anionic sites. Histochem J 16:657–669
Van Kuppevelt THMSM, Cremers FPM, Domen JGW, Kuyper CMA (1984) Staining of proteoglycans in mouse lung alveoli. II. Characterization of the cuprolinic blue-positive, anionic sites. Histochem J 16:671–686
Van Kuppevelt THMSM, Rutten TLM, Kuyper CMA (1987) Ultrastructural localization of proteoglycans in tissue using Cuprolinic blue according to the critical electrolyte concentration method: comparison with biochemical data from the literature. Histochem J 19:520–526
Van Kuppevelt THMSM, Janssen HMJ, Van Beuningen HM, Cheung K, Schijen MMA, Kuyper CMA, Veerkamp JH (1987) Isolation and characterization of a collagen fibril-associated dermatan sulphate proteoglycan from bovine lung. Biochim Biophys Acta 926:296–309
Vinters HV (1987) Cerebral amyloid angiopathy, a critical review. Stroke 18:311–324
Volker W, Schmidt A, Buddecke E (1987) Mapping of proteoglycans in human arterial tissue. Eur J Cell Biol 45:72–79
Wen GY, Wisniewski HM (1987) High resolution analysis of paired helical filaments in Alzheimer's disease. J Electron Microsc Technol 5:347–355
Wight TN, Ross R (1975) Proteoglycans in primate arteries. I. Ultrastructural localization and distribution in the intima. J Cell Biol 67:660–674
Wisniewski HM, Terry RD (1973) Reexamination of the pathogenesis of the senile plaque. In: Zimmerman HM (ed) Progress in neuropathology. Grune and Stratton, New York, pp 1–26
Wisniewski HM, Wen GY (1985) Substructure of paired helical filaments from Alzheimer's disease neurofibrillary tangles. Acta Neuropathol (Berl) 66:173–176
Wong CW, Quaranta V, Glenner GG (1985) Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are genetically related. Proc Natl Acad Sci USA 82:8729–8732
Yamada M, Tsukagoshi H, Otoma E, Hayakawa M (1987) Cerebral amyloid angiopathy in the aged. Neurology 234:371–376
Author information
Authors and Affiliations
Additional information
Supported by the Alzheimer's Disease Research Program of the American Health Assistance Foundation, The Alzheimer's Disease and Related Disorders Association Pilot Grant 87-019 and the Friends of Alzheimer's Research in conjunction with the University of Washington Alzheimer's Disease Research Center
Rights and permissions
About this article
Cite this article
Snow, A.D., Lara, S., Nochlin, D. et al. Cationic dyes reveal proteoglycans structurally integrated within the characteristic lesions of Alzheimer's disease. Acta Neuropathol 78, 113–123 (1989). https://doi.org/10.1007/BF00688198
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00688198