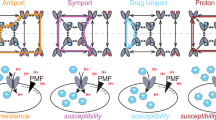
Abstract
Uncoupler resistance presents a potential challenge to the conventional chemiosmotic coupling mechanism. InE. coli, an adaptive response to uncouplers was found in cell growing under conditions requiring oxidative phosphorylation. It is suggested that uncoupler-resistant mutants described in the earlier literature might represent a constitutive state of expression of this “low energy shock” adaptive response. In the environment, bacteria are confronted by nonclassical uncoupling factors such as organic solvents, heat, and extremes of pH. It is suggested that the low energy shock response will aid the cell in coping with the effects of natural uncoupling factors. The genetic analysis of uncoupler resistance has only recently began, and is yielding interesting and largely unexpected results. InBacillus subtilis, a mutation in fatty acid desaturase causes an increased content of saturated fatty acids in the membrane and increased uncoupler resistance. The protonophoric efficiency of uncouplers remains unchanged in the mutants, inviting nonorthodox interpretations of the mechanism of resistance. InE. coli, two loci conferring resistance to CCCP and TSA were cloned and were found to encode multidrug resistance pumps. Resistance to one of the uncouplers, TTFB, remained unchanged in strains mutated for the MDRs, suggesting a resistance mechanism different from uncoupler extrusion.
Similar content being viewed by others
References
Alper, S., Duncan, L., and Losick, R. (1994).Cell 77 195–205.
Aono, R., Aibe, K., Inoue, A., and Horikoshi, K. (1991).Agric. Biol. Chem. 55 1935–1938.
Avetisyan, A. V., Dibrov, P. A., Skulachev, V. P., and Sokolov, M. V. (1989).FEBS Lett. 254 17–21.
Avetisyan, A. V., Bogachev, A. V., Murtasina, R. A., and Skulachev, V. P. (1993).FEBS Lett. 317 267–270.
Bohlmann, H., and Apel, K. (1991).Annu. Rev. Plant Physiol. Plant Mol. Biol. 42 227–240.
Burland, V., Plunkett, G. III, Daniels, D. L., and Blattner, F. R. (1994).Genomics 16 551–561.
Clejan, S., Guffanti, A. A., Falk, L. H., and Krulwich, T. A. (1988).Biochim. Biophys. Acta 932 43–51.
Cohen, S. P., Levy, S. B., Foulds, J., and Rosner, J. L. (1993).J. Bacteriol. 175 7856–7862.
Decker, S. J., and Lang, D. R. (1978).J. Biol. Chem. 253 6738–6743.
Dibrov, P. A. (1991).Biochim. Biophys. Acta 1056 209–224.
Dimroth, P. (1992a). InAlkali Cation Transport Systems in Prokaryotes (Bakker, E. P., ed.), CRC Press, Boca Raton, Florida, pp. 77–100.
Dimroth, P. (1992b). InAlkali Cation Transport Systems in Prokaryotes (Bakker, E. P., ed.), CRC Press, Boca Raton, Florida, pp. 139–154.
Dunkley, E. A., Jr., Clejan, S., and Krulwich, T. A. (1991).J. Bacteriol. 173 7750–7755.
Gage, D. J., and Neidhardt, F. C. (1993).J. Bacteriol. 175 7105–7108.
Guffanti, A. A., and Krulwich, T. A. (1992).J. Biol. Chem. 267 9580–9588.
Guffanti, A. A., Blumenfeld, H., and Krulwich, T. A. (1981).J. Biol. Chem. 256 8416–8421.
Guffanti, A. A., Clejan, S., Falk, L. H., Hicks, D. B., and Krulwich, T. A. (1987).J. Bacteriol. 169 4469–4478.
Inoue, A., and Horikoshi, K. (1989).Nature (London)338 264–265.
Kinoshita, N., Unemoto, T., and Kobayashi, H. (1984).J. Bacteriol. 160 1074–1077.
Krulwich, T. A., and Guffanti, A. A. (1989).J. Bioenerg. Biomembr. 21 663–678.
Krulwich, T. A., Guffanti, A. A., and Seto-Young, D. (1990).FEMS Microbiol. Rev. 75 271–278.
Krulwich, T. A., Quirk, P. G., and Guffanti, A. A. (1990).Microbiol. Rev. 54 52–65.
Lehrer, R. I., Ganz, T., and Selsted, M. E. (1991).Cell 64 229–230.
Levy, S. B. (1992).Antimicr. Agents Chemother. 36 695–703.
Lewis, K. (1994).Trends Biochem. Sci. 19 119–123.
Lomovskaya, O., and Lewis, K. (1992).Proc. Natl. Acad. Sci. USA 89 8938–8942.
Marger, M. D., and Saier, M. H., Jr. (1993).Trends Biochem. Sci. 18 13–20.
McFall, E. (1987). InEscherichia coli and Salmonella typhimurium. Cellular and Molecular Biology. (Neidhard, F. C., ed.) ASM, Washington, DC, pp. 1520–1526.
Mitchell, P. (1966). Glynn Research,Chemiosmotic Coupling in Oxidative and Photosynthesis Phosphorylation. Bodmin.
Nakano, S., and Onoda, T. (1989).J. Basic Microbiol. 29 163–169.
Naroditskaya, V., Schlosser, M. J., Fang, N. Y., and Lewis, K. (1993).Biochem. Biophys. Res. Commun. 196 803–809.
Neiyfakh, A. A., Bidnenko, V. and Chen, L. B. (1991).Proc. Natl. Acad. Sci. USA 88 4781–4785.
Neijssel, O. M., Buurman, E. T., and de Mattos, M. J. T. (1990).Biochim. Biophys. Acta 1018 252–255.
Nikaido, H. (1994).J. Biol. Chem. 269 3905–3908.
Reizer, A., Deutscher, J., Saier, M. H., and Reizer, J. (1991).Mol. Microbiol. 5 1081–1089.
Rosen, B. P. (1986).Methods Enzymol. 125 328–336.
Rottenberg, H. (1990).Biochim. Biophys. Acta 1018 1–17.
Rouch, D. A., Cram, D. S., DiBernardino, D., Littlejohn, G., and Skurray, R. A. (1990).Mol. Microbiol. 4 2051–2062.
Schulein, R., Gentschev, I., Mollenkopf, H. J., and Goebel, W. (1992).Mol. Gen. Genet. 234 155–163.
Sedgwick, E. G., and Bragg, P. D. (1992).Biochim. Biophys. Acta 1099 45–50.
Sedgwick, E. G., Hou, C., and Bragg, P. D. (1984).Biochim. Biophys. Acta 767 479–492.
Sikkema, J., de Bont, J. A. M., and Poolman, B. (1994).J. Biol. Chem. 269 8022–8028.
Sikkema, J., Poolman, B., Konings, W. N., and de Bont, J. A. M. (1992).J. Bacteriol. 174 2986–2992.
Skulachev, V. P. (1989).J. Bioenerg. Biomembr. 21 635–648.
Skulachev, V. P. (1991).FEBS Lett. 294 158–162.
Skulachev, V. P. (1994).Biochim. Biophys. Acta (in press).
Skurray, R. A. (1989).J. Gen. Microbiol. 135 1–103.
Tennent, J. M., Lyon, B. R., Midgley, M., Jones, I. G., Purewal, A. S., and
Terada, H. (1990).Environ Health Persp. 87 213–218.
Terada, H., and van Dam, K. (1975).Biochim. Biophys. Acta 387 507–518.
Tokuda, H. (1992). InAlkali Cation Transport Systems in Prokaryotes, (Bakker, E. P., ed.), CRC Press, Boca Raton, Florida, pp. 125–138.
Westerhoff, H. V., Kell, D. B., Kamp, F., and van Dam, K. (1988). InMicrocompartmentation (Jones, D. P., ed.), CRC Press, Boca Raton, Florida.
Wojtczak, L., and Schonfeld, P. (1993).Biochim. Biophys. Acta 1183 41–57.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lewis, K., Naroditskaya, V., Ferrante, A. et al. Bacterial resistance to uncouplers. J Bioenerg Biomembr 26, 639–646 (1994). https://doi.org/10.1007/BF00831539
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00831539