Summary
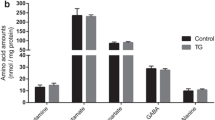
A massive cerebral release of amino acids and ammonia was found in early-onset dementia of Alzheimer type. Aspartate and glycine were liberated in high concentrations, whereas glutamate remained rather unchanged. This excess cerebral protein catabolism is due to a 44% reduction in cerebral glucose metabolism. Whereas glutamate and other glucoplastic amino acids may substitute glucose, elevated aspartate may contribute to neuronal damage. The results are discussed with respect to a possible neuronal insulin/insulin receptor deficiency.
Similar content being viewed by others
References
Bowen DM, White P, Spillane JA, Goodhardt MJ, Curzon G, Iwangoff P, Meier-Ruge W, Davison AN (1979) Accelerated ageing or selective neuronal loss as an important cause of dementia? Lancet I: 11–14
Butcher SP, Sandberg M, Hagberg H, Hamberger A (1987) Cellular origins of endogenous amino acids released into the extracellular fluid of the rat striatum during severe insulin-induced hypoglycemia. J Neurochem 48: 722–728
Cotman CW, Monaghan DT, Ottersen OP, Storm-Mathisen J (1987) Anatomical organization of excitatory amino acid receptors and their pathways. TINS 10: 273–280
Hendricks SA, Roth J, Rishi S, Becker KL (1983) Insulin in the nervous system. In: Krieger DT, Martin JB, Brownstein MJ (eds) Brain peptides. Wiley, New York, pp 903–939
Hoyer S (1970) Der Aminosäurenstoffwechsel des normalen menschlichen Gehirns. Klin Wochenschr 48: 1239–1243
Hoyer S (1988) Metabolism and circulation in normal and abnormal aging processes of the brain. What are the mechanisms of neuronal degeneration? In: Henderson AS, Henderson JH (eds) Etiology of dementia of Alzheimer's type. Dahlem workshop report LS43. Wiley, Chichester (in press)
Hoyer S, Oesterreich K, Wagner O (1988) Glucose metabolism as the site of the primary abnormality in early-onset dementia of Alzheimer type? J Neurol 235: 143–148
Hyman BT, van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science 225: 1168–1170
Jahr CE, Stevens CD (1987) Glutamate activates multiple single channel conductances in hippocampal neurons. Nature 325: 522–525
Johnson JW, Ascher P (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325: 525–531
Kahn CR (1985) The molecular mechanism of insulin action. Ann Rev Med 36: 429–451
Lying-Tunell U, Lindblad BS, Malmlund HO, Persson B (1980) Cerebral blood flow and metabolic rate of oxygen, glucose, lactate, pyruvate, ketone bodies and amino acids. I. Young and old normal subjects. Acta Neurol Scand 62: 265–275
Lying-Tunell U, Lindblad BS, Malmlund HO, Persson B (1981) Cerebral blood flow and metabolic rate of oxygen, glucose, lactate, pyruvate, ketone bodies and amino acids. II. Presenile dementia and normal-pressure hydrocephalus. Acta Neurol Scand 63: 337–350
Maragos WF, Debowey DL, Reiner A, Rustioni A, Penney JB, Young AB (1986) Colocalization of congo red-stained neurofibrillary tangles in glutamate immunoreactive neurons in the hippocampus. Soc Neurosci Abstr 12: 442
Monaghan DT, Holets VR, Toy DW, Cotman CW (1983) Anatomical distributions of four pharmacologically distinct3H-L-glutamate binding sites. Nature 306: 176–179
Moore S, Stein WH (1954) A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem 211: 907–913
Norberg K, Siesjö BK (1976) Oxidative metabolism of the cerebral cortex of the rat in severe insulin-induced hypoglycaemia. J Neurochem 26: 345–352
Pearson RCA, Esiri MM, Hiorns RW, Wilcock GK, Powell TPS (1985) Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA 82: 4531–4534
Procter AW, Palmer AM, Francis PT, Low SL, Neary D, Murphey E, Doshi R, Bowen DM (1988) Evidence of glutamatergic denervation and possible abnormal metabolism in Alzheimer's disease. J Neurochem 50: 790–802
Rothman S (1984) Synaptic release of excitatory amino acid neurotransmitter mediates anoxic neuronal death. J Neurosci 4: 1884–1891
Rothman SM, Thurston JH, Hauhart RE (1987) Delayed neurotoxicity of excitatory amino acids in vitro. Neuroscience 22: 471–480
Sims NR, Bowen DM, Neary D, Davison AN (1983) Metabolic processes in Alzheimer's disease: Adenine nucleotide content and production of14CO2 from (U14-C) glucose in vitro human neocortex. J Neurochem 41: 1329–1334
Sims NR, Blass JP, Murphy C, Bowen DM, Neary D (1987a) Phosphofructokinase activity in the brain in Alzheimer's disease. Ann Neurol 21: 509–510
Sims NR, Finegan JM, Blass JP, Bowen DM, Neary D (1987b) Mitochondrial function in brain tissue in primary degenerative dementia. Brain Res 436: 30–38
Sorbi S, Bird ED, Blass JP (1983) Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol 13: 72–78
Welch BL (1947) The generalization of student's problem when several different variances are involved. Biometrica 34: 28–35
Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FAO (1987) Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121: 1562–1570
Wong KL, Tyce GM (1983) Glucose and amino acid metabolism in rat brain during sustained hypoglycemia. Neurochem Res 8: 401–415
Young WS (1986) Periventricular hypothalamic cells in the rat brain contain insulin mRNA. Neuropeptides 8: 93–97
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoyer, S., Nitsch, R. Cerebral excess release of neurotransmitter amino acids subsequent to reduced cerebral glucose metabolism in early-onset dementia of Alzheimer type. J. Neural Transmission 75, 227–232 (1989). https://doi.org/10.1007/BF01258634
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01258634