Summary
Decreased reduced glutathione (GSH) levels are an early marker of nigral cell death in Parkinson's disease. Depletion of rat brain GSH by intracerebroventricular administration of buthionine sulphoximine (BSO) potentiates the toxicity of 6-hydroxydopamine (6-OHDA) to the nigrostriatal pathway. We have investigated whether thioctic acid can replenish brain GSH levels following BSO-induced depletion and/or prevent 6-OHDA induced toxicity.
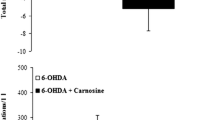
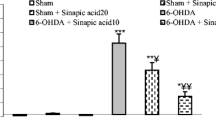
Administration of BSO (2 × 1.6 mg ICV) to rats depleted striatal GSH levels by upto 75%. BSO treatment potentiated 6-OHDA (75 μg ICV) toxicity as judged by striatal dopamine content and the number of tyrosine hydroxylase immunoreactive cells in substantia nigra. Repeated treatment with thioctic acid (50 or 100mg/kg i.p.) over 48h had no effect on the 6-OHDA induced loss of dopamine in striatum or nigral tyrosine hydroxylase positive cells in substantia nigra. Also thioctic acid treatment did not reverse the BSO induced depletion of GSH or prevent the potentiation of 6-OHDA neurotoxicity produced by BSO.
Thioctic acid (50mg or 100mg/kg i.p.) alone or in combination with BSO did not alter striatal dopamine levels but increased dopamine turnover. Striatal 5-HT content was not altered by thioctic acid but 5-HIAA levels were increased.
Under conditions of inhibition of GSH synthesis, thioctic acid does not replenish brain GSH levels or protect against 6-OHDA toxicity. At least in this model of Parkinson's disease, thioctic acid does not appear to have a neuroprotective effect.
Similar content being viewed by others
References
Anderson ME, Meister A (1989) Marked increase in cysteine levels in many regions of the brain after administration of 2-oxo-4-thiazolidine-carboxylate. FASEB J 3: 1632–1636
Aw TY, Wierzbicka G, Jones DP (1991) Oral glutathione increases tissue glutathione concentration in vivo. Chem-Biol Interact 80: 89–97
Busse E, Zimmer G, Schopohl B, Kornhuber B (1992) Influence of α-lipoic acid on intracellular glutathione in vitro and in vivo. Arzneimittelforschung 42: 829–831
Carman LS, Gage FH, Shutts CW (1991) Partial lesion of the substantia nigra: relation between extent of lesion and rotational behaviour. Brain Res 553: 275–283
Cohen G, Heikkila RE (1974) The generation of hydrogen peroxide, superoxide radical and hydroxyl radical by 6-hydroxydopamine, dialuric acid and related cytotoxic agents. J Biol Chem 249: 2447–2452
Cohen G, Spina MB (1989) Deprenyl suppresses the oxidant stress associated with increased dopamine turnover. Ann Neurol 26: 689–690
Dexter DT, Wells FR, Agid F, Agid Y, Jenner P, Marsden CD (1989) Increased nigral iron content and alterations in other metal ions in brain in Parkinson's disease. J Neurochem 52: 1830–1836
Dexter DT, Sian J, Rose S, Hindmarsh JG, Mann VM, Cooper JM, Wells FR, Daniel SE, Lees AJ, Schapira AHV, Jenner P, Marsden CD (1994) Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol 35: 38–44
Ehrenthal W, Preilwitz W (1986) Biochemie und Pharmakologie der Liponsäure. In: Neundörfer B, Sailer D (Hrsg) Interdisziplinäre Bestandsaufnahme der Polyneuropathien. Perimed, Erlangen, S154
Götz ME, Dirr A, Burger R, Janetzky B, Weinmüller M, Chan W, Chen S, Reischmann H, Rausch W-D, Riederer P (1994) Effect of lipoic acid on redox state of coenzyme Q in mice treated with 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine and diethyldithiocarbamate. Eur J Pharmacol 266: 291–300
Greenamyre JT, Garcia-Osuna M, Greene JG (1994) The endogenous cofactors, thioctic acid and dihydrolipoic acid are neuroprotective against NMDA and malonic acid lesions of striatum. Neurosci Lett 171: 17–20
Griffith OW (1982) Mechanism of action, metabolism and toxicity of buthionine sulfoximine and its higher homologues, potent inhibitors of glutathione synthesis. J Biol Chem 257: 13704–13712
Heikkila RE, Cohen G (1973) 6-Hydroxydopamine: evidence for superoxide radicals as an oxidative intermediate. Science 181: 456–457
Jain A, Mårtensson J, Stole E, Auld PA, Méister A (1991) Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci USA 88: 1913–1917
Jenner P, Schapira AHV, Marsden CD (1992) New insights into the cause of Parkinson's disease. Neurology 42: 2241–2250
Kagen V, Shvedova A, Serbinova E, Khan SS, Swanson C, Powell R, Packer L (1992) Dihydrolipoic acid — a universal antioxidant both in the membranes and in the aqueous phase. Reduction of peroxyl, ascorbyl and chromanoxyl. Biochem Pharmacol 44: 1637–1649
Konig JFR, Klippel RA (1963) The rat brain: a stereotaxic atlas of the forebrain and lower parts of the brainstem. Williams & Wilkins, Baltimore
Maitra I, Serbinova E, Tritschler H, Packer L (1995) α-Lipoic acid prevents buthionine sulfoximine-induced cataract formation in newborn rats. Free Rad Biol Med 18: 823–829
Mesina JE, Page RH, Hetzel FW, Chopp M (1989) Administration of L-2-oxo-4-thiazolidine carboxylate increase glutathione levels in rat brain. Brain Res 478: 181–183
Mizuno Y, Matuda S, Yoshino H, Mori H, Hattori N, Ikebe S-I (1994) An immunohistochemical study on α-ketoglutarate dehydrogenase complex in Parkinson's disease. Ann Neurol 35: 204–210
Perry TL, Godin DV, Hansen S (1982) Parkinson's disease: a disorder due to nigral glutathione deficiency. Neurosci Lett 33: 305–310
Pileblad E, Magnusson T (1989) Intracerebroventricular administration of L-buthionine sulfoximine. A method for depleting brain glutathione. J Neurochem 53: 1878–1882
Pileblad E, Magnusson T, Fornstedt B (1989) Reduction of brain glutathione by L-buthionine sulfoximine potentiates the dopamine-depleting action of 6-OHDA in rat striatum. J Neurochem 52: 978–80
Pileblad E, Magnusson T (1992) Increase in rat brain glutathione following intracerebroventricular administration of γ-glutamylcysteine. Biochem Pharmacol 44: 895–903
Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW (1980) High performance liquid chromatography anaylsis of nanomole levels of glutathione, glutathione disulphide and related thiols and disulphides. Anal Biochem 106: 55–62
Riederer P, Sofic E, Rausch W, Schmidt B, Reynolds GP, Jellinger K, Youdim MBH (1989) Transition metals, ferritin, glutathione and ascorbic acid in parkinsonian brains. J Neurochem 52: 515–520
Rose S, Nomoto M, Jackson E, Gibb W, Jenner P, Marsden CD (1989) Transient depletion of nucleus accumbens dopamine content contribute to initial akinesia induced by MPTP in common marmosets. Neuropharmacology 28: 1211–1216
Schapira AHV, Cooper JM, Dexter DT, Jenner P, Clark JB, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem 54: 820–827
Scott BC, Arouma OI, Evans PJ, O'Neill C, Van Der Vliet A, Cross CE, Tritschler H, Halliwell B (1994) Lipoic and dihydrolipoic acids as antioxidants. A critical review. Free Rad Res Commun 20: 119–133
Seaton TA, Jenner P, Marsden CD (1995a) Mitochondrial respiratory enzyme function and superoxide dismutase activity following brain glutathione depletion in the rat. J Neurochem (in press)
Seaton TA, Jenner P, Marsden CD (1995b) The isomers of thioctic acid alter14C-2-deoxyglucose utilisation in rat basal ganglia. Biochem Pharmacol (in press)
Seaton TA, Jenner P, Marsden CD (1995c) Age related changes in14C-2-deoxyglucose incorporation in rat brain produced by the isomers of thioctic acid. Biochem Pharmacol (in press)
Seaton TA, Jenner P, Marsden CD (1995d) Alterations in brain14C-2-deoxyglucose incorporation produced by thioctic acid are dependent on basal glucose utilisation. Biochem Pharmacol (in press)
Shivakumar BR, Ravindranath V (1992) Oxidative stress induced by administration of the neuroleptic drug haloperidol is attentuated by high doses of haloperidol. Brain Res 595: 256–262
Sian J, Dexter DT, Lees AJ, Daniel SE, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994a) Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36: 348–355
Sian J, Dexter DT, Lees AJ, Daniel SE, Jenner P, Marsden CD (1994b) Glutathione-related enzymes in brain in Parkinson's disease. Ann Neurol 36: 356–361
Sofic E, Lange KW, Jellinger K, Riederer P (1992) Reduced and oxidised glutathione in the substantia nigra of patients with Parkinson's disease. Neurosci Lett 142: 128–130
Sumathi R, Kalpana Devi V, Varalakshmi P (1994) DL α-Lipoic acid protection against cadmium-induced tissue lipid peroxidation. Med Sci Res 22: 23–25
Suzuki YJ, Tsuchya M, Packer L (1991) Thioctic acid and dihydrolipoic acid are novel antioxidants which interact with reactive oxygen species. Free Rad Res Commun 15: 255–263
Weiner HJ, Hashim A, Lajtha A, Sershen H (1988) (−)-2-Oxo-4-thiazolidine-carboxylic acid attenuates 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine induced neurotoxicity. Res Commun Abuse 9: 53–60
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seaton, T.A., Jenner, P. & Marsden, C.D. Thioctic acid does not restore glutathione levels or protect against the potentiation of 6-hydroxydopamine toxicity induced by glutathione depletion in rat brain. J. Neural Transmission 103, 315–329 (1996). https://doi.org/10.1007/BF01271243
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01271243