Abstract
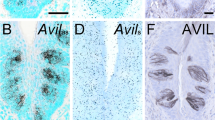
Brush cells are specialised epithelial cells scattered throughout the simple epithelia of the respiratory and alimentary tracts. These cells have been suggested to serve a still unknown receptive function and use nitric oxide as a gaseous messenger molecule. At the light microscope level, brush cells can be identified by antibodies against the actin filament crosslinking proteins villin and fimbrin that not only stain the apical tuft of microvilli and their rootlets, but also label projections emanating from the basolateral surface of these cells. Since brush cells contain numerous intermediate filaments and microtubules and display a complicated basolateral cell morphology, we tested in this study whether antibodies against cytokeratin, tubulin and components of the membrane cytoskeleton might provide further markers for these cells at the light microscope level. Here we show that brush cells (identified by villin antibodies) can be discriminated from the neighbouring simple epithelium of the stomach, pancreatic duct and duodenum by particularly strong immunoreactivity with antibodies specific for cytokeratin 18. Tubulin antibodies reacted strongly with the upper half of brush cells in a pattern not observed in the other epithelial cells of these tissues, including enteroendocrine cells of the duodenum. Ankyrin, a protein that links the spectrin-based membrane cytoskeleton to integral proteins of the plasma membrane was revealed as a third cytoskeleton-associated protein, prominently expressed in brush cells where ankyrin is restricted to the basolateral membrane domain. The apparently high concentration of cytokeratin 18, tubulin and ankyrin in brush cells suggests that these cytoskeletal proteins might play a role in the mechanical stability and polarised organisation of these putative receptor cells.
Similar content being viewed by others
References
Achler C, Filmer D, Merte C, Drenckhahn D (1989) Role of microtubules in polarized delivery of apical membrane proteins to the brush border of the intestinal epithelium. J Cell Biol 109:179–189
Bennett V, Stenbuck PJ (1980) Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem 255:6424–6432
Cetin Y, Kuhn M, Kulaksiz H, Adermann K, Bargsten G, Grube D, Forssmann W-G (1994) Enterochromaffin cells of the digestive system: cellular source of guanylin, a guanylate cyclase activating peptide. Proc Natl Acad Sci USA 91:2935–2939
Davis JQ, Bennett V (1990) The anion exchanger and Na+K+-ATPase interact with distinct sites on ankyrin in in vitro assays. J Biol Chem 265:17252–17256
Drenckhahn D, Bennett V (1987) Polarized distribution of Mr 210000 and 190000 analogs of erythrocyte ankyrin along the plasma membrane of transporting epithelia, neurons and photoreceptors. Eur J Cell Biol 43:479–486
Drenckhahn D, Franz H (1986) Identification of actin, a-actinin and vinculin-containing plaques at the lateral membrane of epithelial cells. J Cell Biol 102:1843–1852
Drenckhahn D, Hofmann HD, Mannherz HG (1983) Evidence for the association of villin with core filaments and rootlets of intestinal epithelial microvilli. Cell Tissue Res 228:409–414
Fujita T, Kanno T, Kobayashi S (1988) The paraneuron. Springer, Heidelberg Berlin New York
Gomi T, Kimura A, Kikushi Y, Higashi K, Tsuchiya H, Sasa S, Kishi K (1991) Electron-microscopic observations of the alveolar brush cell of the rat. Acta Anat 141:294–301
Höfer D, Drenckhahn D (1992) Identification of brush cells in the alimentary and respiratory system by antibodies to villin and fimbrin. Histochemistry 98:237–242
Höfer D, Püschel B, Drenckhahn D (1996) Taste receptor-like cells in the gut identified by expression of α-gustducin. Proc Natl Acad Sci USA (in press)
Kasper M, Höfer D, Woodcock-Mitchell J, Migheli A, Attanasio A, Rudolf T, Müller M, Drenckhahn D (1994) Colocalization of cytokeratin 18 and villin in type III alveolar cells (brush cells) of the rat lung. Histochemistry 101:57–62
Koob R, Zimmermann M, Schoner W, Drenckhahn D (1987) Colocalization and coprecipitation of ankyrin and Na+,K+-ATPase in kidney epithelial cells. Eur J Cell Biol 45:230–237
Koob R, Kraemer D, Trippe G, Aebi U, Drenckhahn D (1990) Association of kidney and parotid Na+,K+-ATPase microsomes with actin and analogs of spectrin and ankyrin. Eur J Cell Biol 53:93–100
Kugler P, Drenckhahn D (1994) Intrinsic source of stomach NO. Nature 370:25–26
Kugler P, Höfer D, Mayer B, Drenckhahn D (1994) Nitric oxide synthase and NADP-linked glucose-6-phosphate dehydrogenase are colocalized in brush cells of rat stomach and pancreas. J Histochem Cytochem 42:1317–1321
Li ZP, Burke EP, Frank JS, Bennett V, Philipson KD (1993) The cardiac Na+−Ca2+ exchanger binds to the cytoskeletal protein ankyrin. J Biol Chem 268:11489–11491
Luciano L, Reale E (1979) A new morphological aspect of the brush cells of the mouse gallbladder epithelium. Cell Tissue Res 201:37–44
Luciano L, Reale E (1990) Brush cells of the mouse gallbladder. Cell Tissue Res 262:339–349
Luciano L, Reale E, Ruska H (1968) Über eine “chemorezeptive” Sinneszelle in der Trachea der Ratte. Z Zellforsch 85:350–375
Luciano L, Castellucci M, Reale E (1981) The brush cells of the common bile duct of the rat. Cell Tissue Res 218:403–420
Major HD, Hampton JC, Rosario B (1961) A simple method for removing the resin from epoxy-embedded tissue. J Biophys Biochem Cytol 9:909–910
Morrow JS, Cianci CD, Ardito T, Mann AS, Kashgarian M (1989) Ankyrin links fodrin to the alpha subunit of Na,K-ATPase in Mardin-Darby canine kidney cells and in intact renal tubule cells. J Cell Biol 108:455–465
Nabeyama A, Leblond CP (1974) “Caveolated cells” characterized by deep surface invaginations and abundant filaments in mouse gastro-intestinal epithelia. Am J Anat 140:147–166
Nelson WJ, Veshnok PJ (1987) Ankyrin binding to (Na+,K+) ATPase and implications for the organization of membrane domains in polarized cells. Nature 328:533–536
Sato A, Miyoshi S (1988) Ultrastructure of the main excretory duct epithelia of the rat parotid and submandibular glands with a review of the literature. Anat Rec 220:230–251
Smith PR, Saccomani G, Joe EH, Angelides KJ, Benos DJ (1991) Amiloride-sensitive sodium channel is linked to the cytoskeleton in renal epithelial cells. Proc Natl Acad Sci USA 88:6971–6975
Srinivasan Y, Elmer L, Davis J, Bennett V, Angelides K (1988) Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature 333:177–183
Trier JS, Allan CH, Marcial MA, Madara JL (1987) Structural features of the apical and tubulovesicular membranes of rodent small intestinal tuft cells. Anat Rec 219:69–77
Tsubouchi S, Leblond CP (1979) Migration and turnover of entero-endocrine and caveolated cells in the epithelium of the descending colon, as shown by radioautography after continuous infusion of3H-thymidine into mice. Am J Anat 156:431–452
Wattel W, Geuze JJ (1978) The cells of the rat gastric groove and cardia. Cell Tissue Res 186:375–391
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. Drs. h.c. Andreas Oksche on the occasion of his 70th birthday
Rights and permissions
About this article
Cite this article
Höfer, D., Drenckhahn, D. Cytoskeletal markers allowing discrimination between brush cells and other epithelial cells of the gut including enteroendocrine cells. Histochem Cell Biol 105, 405–412 (1996). https://doi.org/10.1007/BF01463662
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01463662