Summary
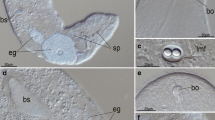
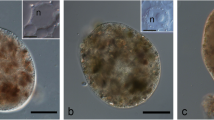
A monoclonal antibody (IIID12) obtained from mice immunized against the entireEntosiphon cytoskeleton highlights the feeding apparatus ofEntosiphon, Peranema, andPloeotia by IF. IGS at the ultrastructural level shows that it labels the cementing material surrounding the microtubular bundles in the three species studied. InEntosiphon additional structures, such as the supplementary plaque, the scaffold structure and the lenticular structure or canal thickening, are also detected by the antibody. Immunoblotting analysis after SDS-PAGE reveals a positive reaction with this antibody to the 58 and 66kDa protein bands inEntosiphon, 82 and 84kDa inPeranema, and 56 and 60kDa inPloeotia. These results demonstrate biochemical homologies in the proteins of the cement material in the three heteronematal eugienoids studied. The possible role of these proteins in microtubule assembly and stabilization is discussed, as well as the role of the cementing material in the mode of the feeding apparatus motion during the ingestion of food.
Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- DMSO:
-
dimethyl sulfoxide
- EDTA:
-
ethylenediaminetetraacetic acid
- IF:
-
immunofluorescence
- IGS:
-
immunogold staining
- HAT:
-
hypoxantin-aminopterinthymidine
- MAb:
-
monoclonal antibody
- MEM:
-
minimum essential medium
- PBS:
-
phosphate buffered saline
- PMSF:
-
phenylmethanesulfonyl fluoride
- TAME:
-
Nα-p-tosyl-L-arginine methyl-ester
- TLCK:
-
Nα-p-tosyl-L-lysine chloromethyl ketone
- SDS-PAGE:
-
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
Bricheux G, Brugerolle G (1986) The membrane cytoskeleton complex of euglenoids. I. Biochemical and immunological characterization of the epiplasmic proteins ofEuglena acus. Eur J Cell Biol 40: 150–159
— — (1987) The pellicular complex of euglenoids. II. Biochemical and immunological comparative study of major epiplasmic proteins. Protoplasma 140: 43–54
Dubreuil RR, Bouck GB (1985) The membrane skeleton of a unicellular organism consists of bridged, articulating strips. J Cell Biol 101: 1884–1896
— — (1988) Interrelationships among the plasma membrane, the membrane skeleton and surface form in a unicellular flagellate. Protoplasma 143: 150–164
—, Rosiere TK, Rosner MC, Bouck GB (1988) Properties and topography of the major integral plasma membrane protein of a unicellular organism. J Cell Biol 107: 191–200
Farmer MA, Triemer RE (1988) A redescription of the genusPloeotia Duj. (Euglenophyceae). Taxon 37: 319–325
Galfré G, Milstein C (1981) Preparation of monoclonal antibodies. Strategies and procedure. Methods Enzymol 73: 3–47
Gallo JM, Schrével J (1985) Homologies between paraflagellar rod proteins from trypanosomes and euglenoids revealed by a monoclonal antibody. Eur J Cell Biol 36: 163–168
—, Karsenti E, Bornens M, Delacourte A, Schrével J (1982) Euglenoid movement inDistigma proteus. II. Presence and localization of an actin-like protein. Biol Cell 44: 149–156
Hilenski LL, Walne PL (1985) Ultrastructure of the flagella of the colorless phagotrophPeranema trichophorum (Euglenophyceae). II. Flagellar roots. J Phycol 21: 125–134
Hoffmann C, Bouck GB (1976) Immunological and structural evidence for patterned intussusceptive surface growth in a unicellular organism. A postulated role for submembranous proteins and microtubules. J Cell Biol 69: 693–715
Hyams JS (1982) TheEuglena paraflagellar rod: structure, relationship to other flagellar components and preliminary biochemical characterization. J Cell Sci 55: 199–210
Kivic PA, Walne PL (1984) An evaluation of a possible phylogenetic relationship between the Euglenophyta and the Kinetoplastida. Origins Life 13: 269–288
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Larsen J, Patterson DJ (1990) Some flagellates (Protista) from tropical marine sediments. J Nat Hist 24: 801–937
Lefort-Tran M, Bré MH, Ranck JL, Pouphile M (1980)Euglena plasma membrane during normal and vitamin B12 starvation growth. J Cell Sci 41: 245–261
Leedale GF (1967) Euglenoid flagellates. Prentice Hall, Englewood Cliffs, NJ
— (1978) Phylogenetic criteria in euglenoid flagellates. BioSystems 10: 183–187
Lonergan TA (1985) Regulation of cell shape inEuglena gracilis. IV. Localization of actin, myosin and calmodulin. J Cell Sci 77: 197–208
Levine ND, Corliss JO, Cox FEG, Deroux G, Grain J, Honigberg BM, Leedale GF, Loeblich AR, Lom J, Lynn D, Merinfeld EG, Page FC, Poljansky G, Sprague V, Vavra J, Wallace FG (1980) A newly revised classification of the protozoa. J Protozool 27: 37–58
Mignot JP (1963) Quelques particularités de l'ultrastructure d'Entosiphon sulcatum (Duj.) Stein, flagellé euglénien. CR Acad Sci Paris 257: 2530–2533
— (1966) Structure et ultrastructure de quelques euglénomonadines. Protistologica 2: 51–117
Miller KR, Miller GJ (1978) Organization of the cell membrane inEuglena. Protoplasma 95: 11–24
Nisbet B (1974) An ultrastructural study of the feeding apparatus ofPeranema trichophorum. J Protozool 21: 39–48
Nahrebne DK, Triemer RE (1990) Proteins involved in movement of the microtubular components of the feeding apparatus inEntosiphon sulcatum. J Protozool 37: abstract 3A-18
O'Farrel PH (1975) High resolution two dimensional electrophoresis of proteins. J Biol Chem 250: 4007–4021
Rosiere TK, Marrs JA, Bouck GB (1990) A 39-kD plasma membrane protein (IP 39) is an anchor for the unusual membrane skeleton ofEuglena gracilis. J Cell Biol 110: 1077–1088
Sogin ML (1989) Evolution of eukaryotic microorganisms and their small subunit ribosomal RNAs. Amer Zool 29: 487–499
—, Gunderson JH (1987) Structural diversity of eukaryotic small subunit ribosomal RNAs. Ann N Y Acad Sci 503: 125–139
Surek B, Melkonian M (1986) A cryptic cytostome is present inEuglena. Protoplasma 133: 39–49
Suzaki T, Williamson RE (1986) Pellicular ultrastructure and euglenoid movement inEuglena ehrenbergii Klebs andEuglena oxyuris Schmarda. J Protozool 33: 165–184
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354
Triemer RE (1986) Light and electron microscopic description of a colorless euglenoid,Serpenomonas costata n.g., n.sp. J Protozool 33: 412–415
—, Fanner MA (1991 a) An ultrastructural comparison of the mitotic apparatus, feeding apparatus, flagellar apparatus and cytoskeleton in euglenoids and kinetoplastids. Protoplasma 164: 91–104
— — (1991 b) The ultrastructural organization of the heterotrophic euglenoids and its evolutionary implications. In: Patterson DJ, Larsen J (eds) The biology of free-living heterotrophic flagellates. Clarendon Press, Oxford, pp 185–203 (The Systematic Association, vol 45)
—, Fritz L (1987) Structure and operation of the feeding apparatus on a colorless euglenoid.Entosiphon sulcatum. J Protozool 34: 39–47
Viguès B, Bricheux G, Metivier C, Brugerolle G, Peck RK (1987) Evidence for common epitopes among proteins of the membrane skeleton of a ciliate, an euglenoid and a dinoflagellate. Eur J Protistol 23: 101–110
Willey RL, Walne PL, Kivic K (1988) Phagotrophy and the origins of the euglenoid flagellates. CRC Crit Rev Plant Sci 7: 303–340
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Belhadri, A., Bayle, D. & Brugerolle, G. Biochemical and immunological characterization of intermicrotubular cement in the feeding apparatus of phagotrophic eugienoids:Entosiphon, Peranema, andPloeotia . Protoplasma 168, 113–124 (1992). https://doi.org/10.1007/BF01666257
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01666257