Abstract
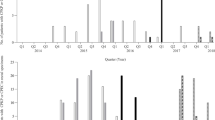
In a Tunisian hospital 27 babies, including 12 who were premature, in a single intensive care unit suffered acute gastroenteritis in the period from January to May 1988. The mean age at the onset of gastroenteritis was 8.4 days; nine babies died.Salmonella wien was isolated from stools (all babies) and blood (4 babies). It was also isolated from the stools of one nurse and from a mattress. Twelve of the babies had received cefotaxime, which was successfully replaced by oral colimycin. The outbreak was stopped by the implementation of infection control measures. All isolates ofSalmonella wien were of the same biotype, and had the same antibiotic resistance pattern (third generation cephalosporins, monobactams, aminoglycosides, chloramphenicol, trimethoprim and sulphonamides) and plasmid DNA restriction pattern. The isolates were all susceptible to a combination of cefotaxime and clavulanic acid (a β-lactamase inhibitor), which displayed synergy, suggesting the presence of a β-lactamase (geometric mean MICs 11.24 µg/ml for cefotaxime alone and 0.24 µg/ml in combination with 0.1 µg/ml potassium clavulanate). All isolates produced TEM-1 and SHV-2 β-lactamase which was not transferable toEscherichia coli by conjugation. The presence of the SHV-2 enzyme inSalmonella wien may allow it to adapt to newer β-lactams which is a cause for concern in this hospital.
Similar content being viewed by others
References
Khemiri F Evaluation desSalmonella de 1977 à 1982. Archives de l'Institut Pasteur de Tunis 1983, 60: 1–2.
Ben Hamed S, Hammami A, Turki A, Kanoun F, Ellouze F, Zribi M Etude de la sensibilité des salmonelles non typhiques isolées à Sfax (Tunisie). Médecine Tropicale 1988, 48: 237–240.
Le Minor L, Grimont PAD Origine et répartition en sérovars des souches deSalmonella isolées en France continentale au cours des années 1984 à 1987. Médecine et Maladies Infectieuses 1989, 19: 12–17.
Philippon A, Fournier G, Cornel E, Paul G, Le Minor L, Névot P Les β-lactamases desSalmonella résistantes à l'ampicilline. Annales de Microbiologie 1984, 135 A: 229–238.
Brun-Buisson C, Legrand P, Philippon A, Montravers F, Ansquer M, Duval J Transferable enzymatic resistance to third generation cephalosporins during nosocomial outbreak of multiresistantKlebsiella pneumoniae. Lancet 1987, ii: 302–306.
Kliebe C, Nies BA, Meyer JF, Tolxdorff-Neutzling RM, Wiedemann B Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrobial Agents and Chemotherapy 1985, 28: 302–309.
Sirot D, Sirot J, Labia R, Morand A, Courvalin P, Darfeuille-Michaud A Transferable resistance to third generation cephalosporins in clinical isolates ofKlebsiella pneumoniae: identification of CTX-1, a novel β-lactamase. Journal of Antimicrobial Chemotherapy 1987, 20: 302–307.
Ben Redjeb S, Ben Yaghlane H, Boujnah A, Philippon A, Labia R Synergy between clavulanic acid and newer β-lactams of nine clinical isolates ofKlebsiella pneumoniae, Escherichia coli, andSalmonella typhimurium resistant to third generation cephalosporins. Journal of Antimicrobial Chemotherapy 1988, 21: 261–269.
Carbonnelle G, Denis F, Marmonier A, Pinon G, Vargues R Bactériologie médicale, techniques usuelles. Simep, Paris, 1987.
Vieu JF, Jeanjean S, Tournier B, Klein B Application à une série unique de bactériophages à la lysotypie deSalmonella serovardublin et deSalmonella serovarenteritidis. Médecine et Maladies Infectieuses 1990, 20: 229–233.
Felix A, Callow BR Paratyphoid-B Vi-phage typing. Lancet 1951, 261: 10–16.
Jarlier V, Nicolas MH, Fournier G, Philippon A Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents inEnterobacteriaceae: hospital prevalence and susceptibility patterns. Reviews of Infectious Diseases 1988, 10: 867–878.
Matthew M, Harris AM, Marshall MJ, Ross GW The use of analytical isoelectric-focusing for detection and identification of β-lactamase. Journal of General Microbiology 1975, 88: 169–175.
Takahashi S, Nagano Y Rapid procedure for isolation plasmid DNA and application to epidemiological analysis. Journal of Clinical Microbiology 1984, 20: 603–618.
McConnell J, Smith HR, Leonardopoulos J, Anderson ES The value of plasmid studies in the epidemiology of infections due to drug-resistantSalmonella wien. Journal of Infectious Diseases 1979, 139: 178–190.
Tauxe RV, Hassan LF, Findeisen KO, Sharrar RG, Blake PA Salmonellosis in nurses: lack of transmission to patients. Journal of Infectious Diseases 1988, 157: 370–373.
Sanders CC, Sanders EW Emergence of resistance during therapy with the newer β-lactam antibiotics: role of inducible β-lactamases and implications for the future. Reviews of Infectious Diseases 1983, 5: 639–648.
Shah PM, Stille EW Escherichia coli andKlebsiella pneumoniae strains more susceptible to cefoxitin than to third generation cephalosporins. Journal of Antimicrobial Chemotherapy 1983, 11: 597–601.
Philippon A, Ben Redjeb S, Fournier G, Ben Hassen A Epidemiology of extended spectrum beta-lactamases. Infection 1989, 17: 347–354.
Philippon A, Labia R, Jacoby GA Extended-spectrum β-lactamases. Antimicrobial Agents and Chemotherapy 1989, 33: 1131–1136.
Legrand P, Fournier G, Buré A, Jarlier V, Nicolas MH, Décré D, Duval J, Philippon A Detection and distribution of extended broad-spectrum beta-lactamases inEnterobacteriaceae in four French hospitals. European Journal of Clinical Microbiology and Infectious Diseases 1989, 8: 527–529.
Petit A, Gerbaud G, Sirot D, Courvalin P, Sirot J Molecular epidemiology of TEM-3 (CTX-1). Antimicrobial Agents and Chemotherapy 1990, 34: 219–224.
Vuthien H, Baudon JJ, Rolland M Salmonellatyphimurium: résistance aux céphalosporines de troisième génération. Médecine et Maladies Infectieuses 1987, 6/7: 398–400.
Barthélémy M, Peduzzi P, Ben Yaghlane H, Labia R Single amino acid substitution between SHV-1 and cefotaxime-hydrolyzing SHV-2 enzyme. FEBS Letters 1988, 231: 217–220.
Shaokat S, Ouellette M, Sirot D, Joly B, Cluzel R Spread of SHV-1 β-lactamase inEscherichia coli isolated from fecal samples in Africa. Antimicrobial Agents and Chemotherapy 1987, 31: 943–945.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hammami, A., Arlet, G., Ben Redjeb, S. et al. Nosocomial outbreak of acute gastroenteritis in a neonatal intensive care unit in tunisia caused by multiply drug resistantSalmonella wien producing SHV-2 beta-lactamase. Eur. J. Clin. Microbiol. Infect. Dis. 10, 641–646 (1991). https://doi.org/10.1007/BF01975816
Issue Date:
DOI: https://doi.org/10.1007/BF01975816