Abstract
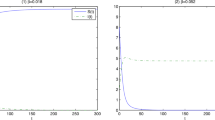
In bacteriophage (phage), rapid and efficient intracellular progeny production is of obvious benefit. A short latent period is not. All else being equal, a longer latent period utilizes host cell resources more completely. Using established parameters of phage growth, a simulation of three successive phage lysis cycles is presented. I have found that high, but not low, host cell densities can select for short phage latent periods. This results from phage with short latent periods more rapidly establishing multiple parallel infections at high host cell concentrations, whereas phage with long latent periods are restricted to growth within a single cell over the same period. This implies that phage with short latent periods habitually grow in environments that are rich in host cells.
Similar content being viewed by others
References
Ackermann H-W, Dubow MS (1987) General properties of bacteriophages. In: Viruses of prokaryotes, vol 1. CRC Press, Boca Raton, Florida
Anderson CW, Eigner J (1971) Breakdown and exclusion of superinfecting T-even bacterio-phage inEscherichia coli. J Virol 8:869–886
Anderson CW, Williamson JR, Eigner J (1971) Localization of parental deoxyribonucleic acid from superinfecting T4 bacteriophage inEscherichia coli. J Virol 8:887–893
Ando A, Furuse K, Watanabe I (1979) Propagation of ribonucleic acid coliphages in gnotobiotic mice. Appl Environ Microbiol 37:1157–1165
Bode W (1967) Lysis inhibition inEscherichia coli infected with bacteriophage T4. J Virol 1:948–955
Bronfenbrenner J, Korb C (1924) Effect of alcohol on the so-called bacteriophage of d'Herrelle. Proc Soc Exp Biol 21:177–179
Chao L, Levin BR, Stewart FM (1977) A complex community in a simple habitat: An experimental study with bacteria and phage. Ecology 58:369–378
Childs JD (1973) Superinfection exclusion by incomplete genomes of bacteriophage T4. J Virol 11:1–8
Cornett JB (1974) Spackle and immunity functions of bacteriophage T4. J Virol 13:312–321
Cummings JH, Jenkins DJA, Wiggins HS (1976) Measurements of the mean transit time of dietary residue through the human gut. Gut 17:210–218
Dennis PP, Bremer H (1974) Differential rate of ribosomal protein synthesis inEscherichia coli B/r. J Mol Biol 94:407–422
Dhillon TS, Dhillon EKS, Chau HC, Li WK, Tsang HC (1976) Studies on bacteriophage distribution: Virulent and temperate bacteriophage content of mammalian feces. Appl Environ Microbiol 32:68–74
Doermann AH (1948) Lysis and lysis inhibition withEscherichia coli bacteriophage. J Bacteriol 55:257–275
Doermann AH (1952) The intracellular growth of bacteriophages I. Liberation of intracellular bacteriophage T4 by premature lysis with another phage or with cyanide. J Gen Physiol 35:645–656
Dulbecco R (1952) Mutual exclusion between related phages. J Bacteriol 63:209–217
Fielding PE, Lunt MR (1970) The relationship between breakdown of superinfecting virus deoxyribonucleic acid and temporal exclusion induced by T4 and T5 bacteriophages. J Gen Virol 6:333–342
Fioramonti J, Bueno L (1980) Motor activity in the large intestine of the pig related to dietary fibre and retention time. Br J Nutr 43:155–162
Freter R (1983) Mechanisms that control the microflora in the large intestine. In: Hertget OJ (ed) Human intestinal flora in health and disease. Academic Press, New York, pp 33–54
Furuse K, Osawa S, Kawashiro J, Tanaka R, Ozawa A, Sawamura S, Yanagawa Y, Nagao T, Watanabe I (1983) Bacteriophage distribution in human faeces: Continuous survey of healthy subjects and patients with internal and leukaemic diseases. J Gen Virol 64:2039–2043
Gear JSS, Brodribb AJM, Ware A, Mann JI (1981) Fibre and bowel transit times, Br J Nutr 45:77–82
Gibbons R, Kapsimalis B (1967) Estimates of the overall rate of growth of the intestinal microflora of hampsters, guinea pigs and mice. J Bacteriol 93:510–512
Guttman B, Kutter E (1983) Overview. In: Mathews CK, Kutter EM, Mosig G, Berget PB (eds) Bacteriophage T4. American Society for Microbiology, Washington, DC, pp 8–10
Hendricks CW (1972) Enteric bacterial growth rates in river water. Appl Microbiol 24:168–174
Horne MT (1970) Coevolutionof Escherichia coli and bacteriophages in chemostat culture. Science 168:992–993
Høverstad T, Jørneklett AB (1984) Short-chain fatty acids and bowel functions in man. Scand J Gastroenterol 19:1059–1065
Josslin R (1970) The lysis mechanism of phage T4: Mutants affecting lysis. Virology 40:719–726
Josslin R (1971) Physiological studies on thet gene defect in T4-infectedEscherichia coli. Virology 44:101–107
Lee A, Gordon J, Lee C-J, Dubos R (1971) The mouse intestinal microflora with emphasis on the strict anaerobes. J Exp Med 133:339–352
Lenski RE (1988) Dynamics of interactions between bacteria and virulent bacteriophage. In: Marshall KC (ed) Advances in microbial ecology, vol 10. Plenum Press, New York, pp 1–44
Lenski RE, Levin BR (1985) Constraints on the coevolution of bacteria and virulent phage: A model, some experiments, and predictions for natural communities. Am Nat 125:585–602
Levin BR, Lenski RE (1983) Coevolution in bacteria and their viruses and plasmids. In: Futuyama DJ, Slatkin M (eds) Coevolution. Sinauer Associates Inc., Sunderland, Massachusetts, pp 99–127
Levin BR, Lenski RE (1985) Bacteria and phage: A model system for the study of the ecology and coevolution of hosts and parasites. In: Ecology and genetics of host-parasite interactions. The Linnean Society of London, pp 227–242
Levin BR, Stewart FM, Chao L (1977) Resource-limited growth, competition, and predation: A model and experimental studies with bacteria and bacteriophage. Am Nat 111:3–24
Levinthal C, Visconti N (1953) Growth and recombination in bacterial viruses. Genetics 38:500–511
Luckey TD (1979) Whither, intestinal microecology. Am J Clin Nutr 32:109–112
May RM, Anderson RM (1983) Parasite-host coevolution. In: Futuyama DJ, Slatkin M (eds) Coevolution. Sinauer Associates Inc., Sunderland, Massachusetts, pp 186–206
McCarthy D, Minner C, Bernstein H, Bernstein C (1976) DNA elongation rates and growing point distributions of wild-type phage T4 and a DNA-delay amber mutant. J Mol Biol 106:963–981
Osawa S, Furuse K, Watanabe I (1981) Distribution of ribonucleic acid coliphages in animals. Appl Environ Microbiol 41:164–168
Rutberg B, Rutberg L (1965) Role of superinfecting phage in lysis inhibition with phage T4 inEscherichia coli J Bacteriol 90:891–894
Sauri CJ, Earhart CF (1971) Superinfection with bacteriophage T4: Inverse relationship between genetic exclusion and membrane association of deoxyribonucleic acid of secondary bacteriophage. J Virol 8:856–859
Savage DC, Dubos R, Schaedler RW (1968) The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med 127:67–76
Smith HW (1965) The development of the flora of the alimentary tract in young animals. J Pathol Bacteriol 90:495–513
Smith HW, Jones JET (1963) Observations on the alimentary tract and its bacterial flora in healthy and diseased pigs. J Pathol Bacteriol 86:387–412
Stent GS (1963) Molecular biology of bacterial viruses. WH Freeman and Co., San Francisco
Stephen AM, Cummings JH (1980a) The microbial contribution to human fecal mass. J Med Microbiol 13:45–56
Stephen AM, Cummings JH (1980b) Mechanism of action of dietary fiber in the human colon. Nature 284:283–284
Stewart FM, Levin BR (1984) The population biology of bacterial viruses: Why be temperate. Theor Pop Biol 26:93–117
Vallée M, de Lapeyrière O (1975) The role of the genesimm ands in the development of immunity against T4 ghosts and exclusion of superinfecting phage inEscherichia coli infected with T4. Virology 67:219–233
Wail AC, Goldberg EB (1973) An extended growth cycle in T4-infectedAerobacter aerogenes. Virology 55:397–399
Yatsudo M, Okamoto K (1973) Immediate-early expression of the gene causing superinfection breakdown in bacteriophage T4B. J Virol 12:1628–1630
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abedon, S.T. Selection for bacteriophage latent period length by bacterial density: A theoretical examination. Microb Ecol 18, 79–88 (1989). https://doi.org/10.1007/BF02030117
Issue Date:
DOI: https://doi.org/10.1007/BF02030117