Abstract
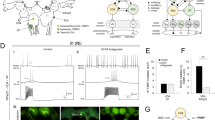
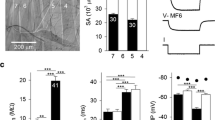
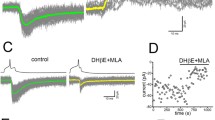
Proteolytic enzymes can have significant effects on the physiological properties of neurons. Although several actions of proteolytic enzymes on the physiology of single neurons have been described, the effects of these enzymes on network properties in the central nervous system (CNS) have received less attention. The effects of bath-applied pronase (0.05%) on synaptic connections and spontaneous activity in theLymnaea CNS were examined. Brief application (i.e. 2–3 min) of pronase modified some, but not all, synapses in the CNS. For example, the chemical synapse between two interneurons, RPeD11 and RPeD1, and between the interneuron, RPeD1, and RPA motoneurons were examined. Both these synapses were either biphasic or monophasic (depolarizing) under control conditions. Pronase exposure eliminated the depolarizing phase of the RPeD11→RPeD1 synapse, but had no effect on the connection between RPeD1 and RPA neurons. In addition, the effects of pronase on electrical-coupling between two peptidergic neurons, VD1 and RPD2, in the CNS were investigated. Pronase decreased the total network input resistance and cell input resistances as well as the steady-state coupling ratio. Furthermore, exposure to pronase induced various changes (i.e. depolarization, hyperpolarization, bursting patterns and afterdischarges) in the activity pattern of different identified neurons in the CNS. Collectively, these data show that even brief exposure to a low concentration of pronase can acutely modify both synapses and neuronal activity.
Similar content being viewed by others
References
Akaike, N., Kaneda, M., Hori, N. and Krishtal, O. A. (1988) Blockade of N-methyl-d-asparate response in enzyme-treated rat hippocampal neurons.Neurosci. Lett.,87, 75–79.
Albuquerque, E. X., Sokoll, M. D., Sonesson, B. and Thesleff, S. (1968) Studies on the nature of the cholinergic receptor.Eur. J. Pharmacol.,4, 40–46.
Allen, C. N., Brady, R., Swann, J., Hori, N. and Carpenter, D. O. (1988) N-methyl-d-aspartate (NMDA) receptors are inactivated by trypsin.Brain Res.,458, 147–50.
Benjamin, P. R. (1983) Gastropod feeding. Behavioural and neuronal analysis of a complex multicomponent system. InNeural Origin of Rhythmic Movements (Soc. Exp. Biol. Symp. 37), ed. A. Roberts and B. L. Roberts, Cambridge, Cambridge University Press, pp. 159–93.
Benjamin, P. R. and Winlow, W. (1981) The distribution of three wide-acting synaptic inputs to identified neurons in the isolated brain ofLymnaea stagnalis (L.).Comp. Biochem. Physiol.,70A, 293–307.
Benjamin, P. R. and Pilkington, J. B. (1986) The electrotonic location of low-resistance intercellular junctions between a pair of giant neurones in the snailLymnaea.J. Physiol.,370, 111–26.
Bennett, M. V. L. (1966) Physiology of electrotonic junctions.Ann. N.Y. Acad. Sci.,157, 509–539.
Berry, M. S. and Pentreath, V. W. (1975) The integrative properties of electrotonic synapses.Comp. Biochem. Physiol.,57A, 589–612.
Berry, M. S. and Pentreath, V. W. (1976) Criteria for distinguishing between monosynaptic and polysynaptic transmission.Brain Res.,105, 1–20.
Betz, W. and Sakmann, B. (1971) ‘Disjunction’ of frog neuromuscular synapses by treatment with proteolytic enzymes.Nature New Biol.,232, 94–95.
Betz, W. and Sakmann, B. (1973) Effects of proteolytic enzymes on function and structure of frog neuromuscular junctionsJ. Physiol.,230, 673–88.
Bulloch, A. G. M. (1987). Somatostatin enhances neurite out-growth and electrical-coupling of regenerating neurons inHelisoma.Brain Res.,412, 6–17.
Burger, M. M. (1969) A difference in the architecture of the surface membrane of normal and virally-transformed cells.Proc. Natl. Acad. Sci. USA,62, 994–1001.
Burger, M. M. (1970) Proteolytic enzymes initiating cell division and escape from contact inhibition of growth.Nature,227, 170–71.
Chen, C. F., von Baumgarten, R. and Takeda, R. (1971) Pacemaker properties of completely isolated neurones inAplysia californica.Nature New Biol.,233, 27–29.
Church, J. and Baimbridge, K. G. (1991) Exposure to high pH medium increases the incidence as extent of dye coupling between rat hippocampal CA1 pyramidal neuronsin vitro J. Neurosci.,11, 3289–3295.
Clapham, A. H., Shrier, A. and De Haan, R. L. (1980) Junctional resistance and action potential delay between embryonic heart cell aggregates.J. Gen. Physiol.,75, 633–54.
Elliott, C. J. and Benjamin, P. R. (1989) Esophageal mechanoreceptors in the feeding system of the pond snailLymnaea stagnalis.J. Neurophysiol.,61, 727–36.
Fain, J. N. and Loken, S. C. (1969) Response of trypsin-treated brown and white fat cells to hormones. Preferential inhibition of insulin action.J. Biol. Chem.,244, 3500–506.
Ferguson, G. P. and Benjamin, P. R. (1991a). The whole-body with-drawal response ofLymnaea stagnalis. I. Identification of central motoneurones and muscles.J. Exp. Biol.,158, 63–95.
Ferguson, G. P. and Benjamin, P. R. (1991b) The whole-body with-drawal response ofLymnaea stagnalis. II. Activation of central motoneurones and muscles by sensory input.J. Exp. Biol.,158, 97–116.
Gadotti, D., Bauce, L. G., Lukowiak, K. and Bulloch, A. G. M. (1986) Transient depletion of seretonin in the nervous system ofHelisoma.J. Neurobiol.,17, 431–47.
Getting, P. A. and Willows, A. O. D. (1974) Modification of neuron properties by electronic synapses. II. Burst formation by electrotonic synapses.J. Neurophysiol.,37, 858–68.
Gu, Q. and Moss, R. L. (1996) 17b-estradiol potentiates kainate-induced currents via activation of the cAMP cascade.J. Neurosci.,16, 3620–29.
Hall, Z.W. and Kelly, R.B. (1971) Enzymatic detachment of end-plate acetylcholinesterase from muscle.Nature New Biol.,232, 62–63.
Hermann, P. M., Ter Maat, A. and Jansen, R. F. (1994) The neural control of egg-laying behaviour in the pond snailLymnaea stagnalis: motor control of shell turning.J. Exp. Biol.,197, 79–99.
Hermann, P. M., Lukowiak, K., Wildering, W. C. and Bulloch, A. G. M. (1997a) Pronase acutely modifies high-voltage-activated Ca2+ current and integrative properties ofLymnaea neurons.Eur. J. Neurosci.,9, 2624–33.
Hermann, P. M., De Lange, R. P. J., Pieneman, A. W., Ter Maat, A. and Jansen, R. F. (1997b) The role of neuropeptides encoded on the CDCH-1 gene in the organization of egg-laying behavior in the pond snailLymnaea stagnalis.J. Neurophysiol.,78, 2859–69.
Inoue, T., Takasaki, M., Lukowiak, K. and Syed, N. I. (1996) Inhibition of the respiratory pattern generating neurons by an identified whole body withdrawal interneuron ofLymnaea stagnalis.J. Exp. Biol.,199 1887–98.
Johnson, B. D. and Byerly, L. (1993) A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+.Neuron,10, 797–804.
Kono, T. (1969) Destruction of insulin effector system of adipose tissue cells by proteolytic enzymes.J. Biol. Chem.,244, 1772–78.
Lee, K. S., Akaike, N. and Brown, A. M. (1977) Trypsin inhibits the action of tetrodotoxin on neurones.Nature,265, 751–53.
Li, K. W., Geraerts, W. P. M. and Joosse, J. (1992) Purification and chemical characterization of caudodorsal cell hormone-II from the egg-laying controlling caudodorsal cells ofLymnaea stagnalis.Peptides,13, 215–20.
Magoski, N. S., Syed, N. I. and Bulloch, A. G. M. (1994) A neuronal network from the molluscLymnaea stagnalis.Brain Res.,645, 201–14.
Magoski, N. S. and Bulloch, A. G. M. (1995) Plasticity of the sign of synaptic transmission between interneurons from the molluscLymnaea stagnalis.Soc. Neurosci. Abstr.,21, 168.
Moroz, L. L., Park J.-H. and Winlow, W. (1993) Nitric oxide activates buccal motor patterns inLymnaea stagnalis.Neuroreport,4, 643–46.
Norekian, T. P. and Satterlie, R. A. (1995) An identified cerebral interneuron initiates different elements of prey capture behavior in the pteropod mollusc,Clione limacina.Invertebrate Neurosci.,1, 235–48.
Park, J.-H. and Winlow, W. (1993) Variant synaptic connections in the respiratory central pattern generator in semi-intact preparations ofLymnaea. J. Physiol.,473, 198P.
Poste, G. (1971) Tissue dissociation with proteolytic enzymes. Adsorption and activity of enzymes at the cell surface.Exp. Cell Res.,65, 359–67.
Rodbell, M., Birnbaumer, L. and Pohl, S. L. (1970) Adenyl cyclase in fat cells. III. Stimulation by secretin and the effects of trypsin on the receptors for lipolytic hormones.J. Biol. Chem.,245, 718–22.
Roubos, E. W. and Van Heumen, W. R. A. (1994) Peptide processing and release by the neuroendocrine cells ofLymnaea stagnalis during an egg laying cycle.Brain Res.,644, 83–89.
Sefton, B. M. and Rubin, H. (1970) Release from density-dependent growth inhibition by proteolytic enzymes.Nature,227, 843–45.
Sketelj, J. and Brzin, M. (1977) Increase in the apparent AChE activity in the mouse diaphragm induced by proteolytic treatment.J. Neurochem.,29, 109–14.
Small, D. L. and Morris, C. E. (1995) Pore properties ofLymnaea stagnalis neuron stretch-activated K+ channelsJ. Exp. Biol.,198, 1919–29.
Soffe, S. R. and Benjamin, P. R. (1980) Morphology of two-electrotonically-coupled giant neurosecretory neurons in the snailLymnaea stagnalis Comp. Biochem. Physiol.,67A, 35–46.
Sokal, R. R. and Rohlf, F. J. (1981)Biometry, San Francisco: W.H. Freeman and Co.
Spencer, G. E. and Winlow, W. (1994) Variant synaptic connections of an identified peptidergic neurone ofLymnaea stagnalis. J. Physiol.,475, 150P.
Syed, N. I. and Winlow, W. (1991a) Respiratory behavior in the pond snailLymnaea stagnalis. I. Behavioral analysis and the identification of motor neurons.J. Comp. Physiol. A,169 541–55.
Syed, N. I. and Winlow, W. (1991b) Respiratory behavior in the pond snailLymnaea stagnalis. II. Neural elements of the central pattern generator (CPG).J. Comp. Physiol. A,169, 557–68.
Ter Maat A., Lodder, J. C. and Wilbrink, M. (1983) Induction of egg-laying in the pond snailLymnaea stagnalis by environmental stimulation of the release of ovulation hormone from the caudo-dorsal cells.Int. J. Invert. Reprod.,6, 239–47.
Ter Maat, A., Dijcks, F. A. and Bos, N. P. A. (1986).In vivo recordings of neuroendocrine cells (Caudo-Dorsal cells) in the pond snail.J. Comp. Physiol. A,158, 853–59.
Trudeau, L. and Castellucci, V. F. (1995) Postsynaptic modifications in long-term facilitation inAplysia: upregulation of excitatory amino acid receptors.J. Neurosci.,15, 1275–84.
Wildering, W. C. and Janse, C. (1992) Serotonergic modulation of junctional conductance in an identified pair of neurons in the molluscLymnaea stagnalis.Brain Res.,595, 343–52.
Winer, B. J., Brown, D. R. and Michels, K. M. (1991)Statistical Principles in Experimental Design. New York: McGraw-Hill.
Winlow, W., Haydon, P. G. and Benjamin, P. R. (1981) Multiple postsynaptic actions of the giant dopamine-containing neuron RPeD1 ofLymnaea stagnalis J. Exp. Biol.,94, 137–148.
Winlow, W. and Haydon, P. G. (1986) A behavioral and neuronal analysis of the locomotory system ofLymnaea stagnalis Comp. Biochem. Physiol.,83A, 13–21.
Yamada, N. and Bilkey, D. K. (1993) Induction of trypsin-induced hyperexcitability in the rat hippocampal slice is blocked by the N-methyl-d-aspartate receptor antagonist, MK-801.Brain Res.,624, 336–38.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hermann, P.M., Bulloch, A.G.M. Pronase modifies synaptic transmission and activity of identifiedLymnaea neurons. Invertebrate Neuroscience 3, 295–304 (1998). https://doi.org/10.1007/BF02577689
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02577689