Abstract.
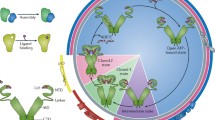
Heat-shock protein 90 (Hsp90) is an abundant and highly conserved molecular chaperone that is essential for viability in eukaryotes. Hsp90 fulfills a housekeeping function in contributing to the folding, maintenance of structural integrity and proper regulation of a subset of cytosolic proteins. A remarkable proportion of its substrates are proteins involved in cell cycle control and signal transduction. Hsp90 acts with a cohort of Hsp90 co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function. The large conformational flexibility of Hsp90 and a multitude of dynamic co-chaperone complexes contribute to generating functional diversity, and allow Hsp90 to assist a wide range of substrates.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. CMLS, Cell. Mol. Life Sci. 59, 1640–1648 (2002). https://doi.org/10.1007/PL00012491
Issue Date:
DOI: https://doi.org/10.1007/PL00012491