Abstract
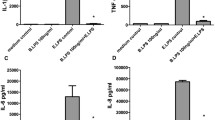
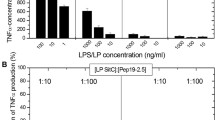
Lipopolysaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria, can be beneficial to the host by activating the innate immune system, or harmful, by inducing inflammation, disseminated intravascular coagulation, multiple organ failure, shock and often death. On the bacteria, and in host biological fluids and cells, LPS is never free but constantly attached to cognate-binding proteins. Understanding how LPS is transported and further recognized by sensors able to deliver a signal, or by inactivating molecules able to neutralize its biological effects, is an important goal. This review describes the large panel of peptides and proteins reported to associate with LPS, and provides information on their origin, their structure and the location of amino acid residues involved in their interaction with LPS. A better understanding of the mode of recognition of LPS by cognate proteins prompted many laboratories to design on a rational basis synthetic molecules which can be used to detect low amounts of endotoxin, or to act as efficient blockers of in vitro and in vivo responses to LPS.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 15 January 2004; received after revision 20 February 2004; accepted 25 February 2004
Rights and permissions
About this article
Cite this article
Chaby, R. Lipopolysaccharide-binding molecules: transporters, blockers and sensors. CMLS, Cell. Mol. Life Sci. 61, 1697–1713 (2004). https://doi.org/10.1007/s00018-004-4020-4
Issue Date:
DOI: https://doi.org/10.1007/s00018-004-4020-4