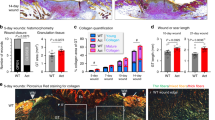
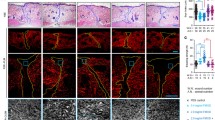
Abstract
Repair of wounds usually results in restoration of organ function, even if suboptimal. However, in a minority of situations, the healing process leads to significant scarring that hampers homeostasis and leaves the tissue compromised. This scar is characterized by an excess of matrix deposition that remains poorly organized and weakened. While we know much of the early stages of the repair process, the transition to wound resolution that limits scar formation is poorly understood. This is particularly true of the inducers of scar formation. Here, we present a hypothesis that it is the matrix itself that is a primary driver of scar, rather than being simply the result of other cellular dysregulations.



Similar content being viewed by others
References
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341:738–746
Harty M, Neff AW, King MW, Mescher AL (2003) Regeneration or scarring: an immunologic perspective. Dev Dyn 226:268–279
Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326:1216–1219
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 45:314–321
Trent JT, Kirsner RS (2006) Wounds and malignancy. Adv Skin Wound Care 16:31–34
Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E (2004) Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118:635–648
Nelson CM, Bissell MJ (2006) Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 22:287–309
Liu Y, Min D, Bolton T et al (2009) Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 32:117–119
James GA, Swogger E, Wolcott R et al (2008) Biofilms in chronic wounds. Wound Repair Regen 16:37–44
Wynn T (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Werner S, Krieg T, Smola H (2007) Keratinocyte–fibroblast interactions in wound healing. J Invest Dermatol 127:998–1008
Martin P (1997) Wound healing—aiming for perfect skin regeneration. Science 276:75–81
Adzick NS, Harrison MR, Glick PL, Beckstead JH, Villa RL, Scheuenstuhl H, Goodson WH (1985) Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg 20:315–319
Yamaguchi Y, Yoshikawa K (2001) Cutaneous wound healing: an update. J Dermatol 28:521–534
Schultz G (2008) Dynamic reciprocity—how cells and extracellular matrix communicate to heal wounds. Third Congress of the World Union of Wound Healing Societies, Canada
Eckes B, Nischt R, Krieg T (2010) Cell–matrix interactions in dermal repair and scarring. Fibrogenesis Tissue Repair 3:4
Widgerow AD, Chait LAC, Stals R, Stals P, Candy G (2009) Multimodality scar management program. Aesthetic Plast Surg 33:533
Siebert JW, Burd AR, McCarthy JG, Weinzweig J, Ehrlich HP (1990) Fetal wound healing: a biochemical study of scarless healing. Plast Reconstr Surg 85:495–504
Woolley K, Martin P (2000) Conserved mechanisms of repair: from damaged single cells to wounds in multicellular tissues. Bioessays 22:911–919
Coolen NA, Schouten KC, Middelkoop E et al (2010) Comparison between human fetal and adult skin. Arch Dermatol Res 302:47–55
Lorenz HP, Longaker MT, Perkocha LA et al (1992) Scarless wound repair: a human fetal skin model. Development 114:253–259
Tran KT, Lamb P, Deng JS (2004) Matrikines and matricryptins: implications for cutaneous cancers and skin repair. J Dermatol Sci 40:11–20
Tran KT, Griffith L, Wells A (2004) Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen 12:262–268
Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V (1997) Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277:225–228
Aarabi S, Longaker MT, Gurtner GC (2007) Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med 4:e234
Niessen FB, Spauwen PH, Schalkwijk J, Kon M (2008) On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 104:1435–1458
Martin P, Parkhurst SM (2004) Parallels between tissue repair and embryo morphogenesis. Development 131:3021–3034
Martin P, Lewis J (1992) Actin cables and epidermal movement in embryonic wound healing. Nature 360:179–183
Colwell AS, Longaker MT, Lorenz H (2003) Fetal wound healing. Front Biosci 8:s1240–s1248
ChenW FuX, Ge S, Sun T, Zhou G, Jiang D, Sheng Z (2005) Ontogeny of expression of transforming growth factor-beta and its receptors and their possible relationship with scarless healing in human fetal skin. Wound Repair Regen 13:68–75
Ferguson MW, O’Kane S (2004) Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B 359:839–850
Armour A, Scott PG, Tredget EE (2007) Cellular and molecular pathology of HTS: basis for treatment. Wound Repair Regen 15:S6–S17
Mehrad B, Keane MP, Gomperts BN, Strieter RM (2007) Circulating progenitor cells in chronic lung disease. Expert Rev Respir Med 1:157–165
Zeisberg EM, Tarnavski O, Zeisberg M et al (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13:952–961
Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R (2007) Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 282:23337–23347
National Heart Blood Institute Lung Mortality Morbidity (2002) Chart book on cardiovascular, lung, and blood diseases. US Department of Health and Human Services, Bethesda
Babu M, Wells A (2002) Dermal–epidermal communication in wound healing. Wounds 200113:183–189
Neilson EG (2006) Mechanisms of disease fibroblasts—a new look at an old problem. Nat Clin Pract Nephrol 2:101–108
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3:349–363
Eckes B, Krieg T (2004) Regulation of connective tissue homeostasis in the skin by mechanical forces. Clin Exp Rheumatol 22:S73–S76
Rivera J, Lozano ML, Navarro-Nunez L, Vicente V (2009) Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica 94:700–711
Nieswandt B, Varga-Szabo D, Elvers M (2009) Integrins in platelet activation. J Thromb Haemost 7:206–209
Theilgaard-Monch K, Knudsen S, Follin P, Borregaard N (2004) The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol 172:7684–7693
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83:835–870
Clark RA (1993) Biology of dermal wound repair. Dermatol Clin 11:647–666
Greiling D, Clark RA (1997) Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci 110:861–870
McDonald JA, Kelley DG, Broekelmann TJ (1982) Role of fibronectin in collagen deposition: fab’ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol 92:485–495
Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, Roers A, Eming SA (2010) Differential roles of macrophages in diverse phases of skin repair. J Immunol 184:3964–3977
Roberts AB, Heine UI, Flanders KC, Sporn MB (1990) Transforming growth factor-beta. Major role in regulation of extracellular matrix. Ann NY Acad Sci 580:225–232
Raab G, Klagsbrun M (1997) Heparin-binding EGF-like growth factor. Biochim Biophys Acta 1333:F179–F199
Marikovsky M, Breuing K, Liu PY et al (1993) Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci USA 90:3889–3893
Tonnesen MG, Feng X, Clark RA (2000) Angiogenesis in wound healing. J Investig Dermatol Symp Proc 5:40–46
Herrick SE, Sloan P, McGurk M, Freak L, McCollum CN, Ferguson MW (1992) Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol 141:1085–1095
Flaumenhaft R, Rifkin DB (1991) Extracellular matrix regulation of growth factor and protease activity. Curr Opin Cell Biol 3:817–823
Baneyx G, Baugh L, Vogel V (2002) Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci USA 99:5139–5143
Yan C, Grimm WA, Garner WL et al (2010) Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol 176:2247–2258
Spaeth EL, Dembinski JL, Sasser AK et al (2009) Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 4:e4992
Papetti M, Herman IM (2002) Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol 282:C947–C970
Iozzo RV, Schaefer L (2010) Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J 277:3864–3875
Hynes RO (1999) The dynamic dialogue between cells and matrices: implications of fibronectin’s elasticity. Proc Natl Acad Sci USA 96:2588–2590
Clark RA (1990) Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol 94:128S–134S
Schultz GS, Wysocki A (2009) Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 17:153–162
Heino J (2007) The collagen family members as cell adhesion proteins. Bioessays 29:1001–1010
Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S (2004) CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol 25:201–209
Liu L, Callahan MK, Huang D, Ransohoff RM (2005) Chemokine receptor CXCR3: an unexpected enigma. Curr Top Dev Biol 68:149–181
Clark RA (1993) Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci 306:42–48
Juhasz I, Murphy GF, Yan HC, Herlyn M, Albelda S (1993) Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. Am J Pathol 143:1458–1469
Werner S, Krieg T, Smola H (2001) Keratinocyte–fibroblast interactions in wound healing. J Invest Dermatol 127:998–1008
Vuorio E, de Crombrugghe B (1990) The family of collagen genes. Annu Rev Biochem 59:837–872
Jaffe AT, Heymann WR, Lawrence N (1995) Epidermal maturation arrest. Dermatol Surg 25:900–903
Gipson IK, Spurr-Michaud SJ, Tisdale AS (1988) Hemidesmosomes and anchoring fibril collagen appear synchronously during development and wound healing. Dev Biol 126:253–262
Shiraha H, Gupta K, Drabik KA, Wells A (2000) Aging fibroblasts present reduced epidermal growth factor (EGF) responsiveness due to preferential loss of EGF receptors. J Biol Chem 275:19343–19351
Chen P, Gupta K, Wells A (1994) Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J Cell Biol 124:547–555
Shiraha H, Glading A, Wells A (2002) Activation of M-calpain (calpain II) by epidermal growth factor is limited by PKA phosphorylation of M-calpain. Mol Cell Biol 22:2716–2727
Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE (2003) Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol 162:149–160
Wierzbicka-Patynowski I, Schwarzbauer JE (2003) The ins and outs of fibronectin matrix assembly. J Cell Sci 116:3269–3276
Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S (2002) Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem 277:37377–37381
Iyer AK, Tran KT, Borysenko CW, Cascio M, Camacho CJ, Blair HC, Bahar I, Wells A (2007) Tenascin cytotactin epidermal growth factor-like repeat binds epidermal growth factor receptor with low affinity. J Cell Physiol 211:748–758
Iyer AK, Tran KT, Griffith L, Wells A (2008) Cell surface restriction of EGFR by a tenascin cytotactin-encoded EGF-like repeat is preferential for motility-related signaling. J Cell Physiol 214:504–512
Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, Wells A (2001) Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol 154:459–468
Chiquet-Ehrismann R, Chiquet M (2003) Tenascins: regulation and putative functions during pathological stress. J Pathol 200:488–499
Trebaul A, Chan EK, Midwood KS (2007) Regulation of fibroblast migration by tenascin-C. Biochem Soc Trans 35:695–697
Mann K, Deutzmann R, Aumailley M, Timpl R, Raimondi L, Yamada Y, Pan TC, Conway D, Chu ML (1989) Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV and cells. EMBO 8:65–72
Chakravarti S, Tam MF, Cheung AE (1990) The basement membrane glycoprotein entactin promotes cell attachment and binds calcium. J Biol Chem 265:10597–10603
Merline R, Schaefer RM, Schaefer L (2009) The matricellular functions of small leucine-rich proteoglycans (SLRPs). J Cell Commun Signal 3:323–335
Reed CC, Iozzo RV (2002) The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J 19:249–255
Zhang Z, Li XJ, Liu Y, Zhang X, Li YY, Xu WS (2007) Recombinant human decorin inhibits cell proliferation and downregulates TGF-beta1 production in hypertrophic scar fibroblasts. Burns 33:634–641
Krishna P, Regner M, Palko J, Liu F, Abramowitch S, Jiang J, Wells A (2010) The effects of decorin and HGF-primed vocal fold fibroblasts in vitro and ex vivo in a porcine model of vocal fold scarring. Laryngoscope 120:2247–2257
Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, Tensen CP (2001) Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol 194:398–405
Tensen CP, Flier J, van der Raaij-Helmer EM et al (1999) Human IP-9. A keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3). J Invest Dermatol 112:716–722
Satish L, Yager D, Wells A (2003) ELR-negative CXC chemokine IP-9 as a mediator of epidermal–dermal communication during wound repair. J Invest Dermatol 120:1110–1117
Yates CC, Whaley D, Kulasekeran P, Hancock WW, Lu B, Bodnar R, Newsome J, Hebda PA, Wells A (2007) Delayed and deficient dermal mat-uration in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol 171:484–495
Yates CC, Whaley D, Yen A, Kulesekaran P, Hebda PA, Wells A (2008) ELR-negative CXC chemokine CXCL11(IP-9/I-TAC) facilitates dermal and epidermal maturation during wound repair. Am J Pathol 173:643–652
Bodnar R, Yates C, Wells A (2006) IP-10 blocks VEGF-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res 98:617–625
Bodnar RJ, Yates CC, Du X, Wells A (2009) ELR-negative chemokine IP-10/CXCL10 induces dissociation of newly-formed vessels secondary to calpain cleavage of beta3 integrin. J Cell Sci 122:2064–2077
Shiraha H, Glading A, Chou J, Jia Z, Wells A (2002) Activation of m-calpain (calpain II) by epidermal growth factor is limited by PKA phosphorylation of m-calpain. Mol Cell Biol 22:2716–2720
Yates CC, Whaley D, Hooda S, Hebda PA, Bodnar RJ, Wells A (2009) Delayed re-epithelialization and basement membrane regeneration after wounding in mice lacking CXCR3. Wound Repair Regen 17:34–41
Yates CC, Krishna P, Whaley D, Bodnar R, Turner T, Wells A (2010) Lack of CXC chemokine receptor 3 signaling leads to hypertrophic and hypercellular scarring. Am J Pathol 176:1743–1755
Stramer BM, Mori R, Martin P (2007) The inflammation–fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol 127:1009–1017
Raghow R (1994) The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J 8:823–831
Acknowledgments
These studies were supported by grants from the National Institute of General Medical Science of the National Institutes of Health (USA) (GM063569). Services in kind were provided by the Pittsburgh VA Medical Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yates, C.C., Bodnar, R. & Wells, A. Matrix control of scarring. Cell. Mol. Life Sci. 68, 1871–1881 (2011). https://doi.org/10.1007/s00018-011-0663-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0663-0