Abstract
Clostridium difficile toxin A causes acute colitis associated with intense infiltration of neutrophils. Although C. difficile toxin A is known to induce nuclear factor-kappaB-mediated chemokine expression in intestinal epithelial cells, little is known about its effect on the regulation of activator protein-1 (AP-1) by mitogen-activated protein kinase (MAPK). In the present study, we investigated whether the MAPK and AP-1 signaling pathway is involved in interleukin (IL)-8 expression and enteric inflammation in response to stimulation with toxin A. Toxin A activated MAPK and AP-1 composed of c-Jun/c-Fos heterodimers in primary intestinal epithelial cells and HT-29 cell lines. Transfection with mutant genes for Ras, c-Jun, p38, or c-Jun N-terminal kinase (JNK) significantly inhibited C. difficile toxin A-induced activation of AP-1 and expression of IL-8 in HT-29 cell lines. Furthermore, the p38 inhibitor SB203580 attenuated toxin A-induced inflammation in vivo in the mouse ileum, evidenced by a significant decrease in neutrophil infiltration, villous destruction, and mucosal congestion. Our results suggest that the Ras/MAPK cascade acts as the upstream signaling for AP-1 activation and IL-8 expression in toxin A-stimulated intestinal epithelial cells and may be involved in the development of enteritis after infection with toxin A-producing C. difficile.
Similar content being viewed by others
Introduction
Clostridium difficile causes antibiotic-associated diarrhea and pseudomembranous colitis in humans. These manifestations are mainly caused by two exotoxins, toxin A (308 kDa) and toxin B (269 kDa), produced by pathogenic strains of C. difficile [1]. In animal models, toxin A causes fluid secretion, mucosal edema, and villous disruption through the induction of massive acute inflammation with neutrophil infiltration [1, 2]. As the first host cells that C. difficile toxins interact with are epithelial cells in the colon [3], it has been proposed that mucosal inflammatory signals may be initiated from intestinal epithelial cells. Working from this hypothesis, several reports have demonstrated that intestinal epithelial cells can produce chemokines, including interleukin (IL)-8, growth-related oncogene-α, and monocyte chemotactic protein-1, in response to C. difficile toxin A [4–7]. However, the pathogenic mechanism of toxin A-induced inflammation has not been fully investigated.
Mucosal inflammation is mediated by chemokines that trigger proinflammatory signals. Toxigenic C. difficile-infected colitis is accompanied by a massive infiltration of neutrophils into the mucosa [1]. CXC chemokines, characterized by two cysteine residues separated by any other amino acid, seem to play important roles in mobilizing inflammatory cells to areas of immune challenge [8], and IL-8 is a CXC chemokine that plays a role in recruiting and activating neutrophils in the intestinal mucosa. This process is primarily controlled at the transcriptional level, and binding sites for the inducible transcription factors, such as nuclear factor-kappaB (NF-κB) and activator protein-1 (AP-1), are present in the promoter region of the IL-8 gene [9]. Although the importance of NF-κB in toxin A-induced chemokine production is apparent [7, 10, 11], it remains unclear how toxin A activates transcription factor AP-1 and induces chemokine expression.
Mitogen-activated protein kinases (MAPKs) comprise an important group of serine- and threonine-signaling kinases that transduce a variety of extracellular stimuli through a cascade of protein phosphorylation, leading to the activation of transcription factors [12–14]. Three groups of MAPKs have been identified in mammalian cells: (1) p42 and p44 extracellular signal-regulated kinase (ERK), (2) p38 MAPK with α, β, γ, and δ isoforms, and (3) p46 and p54 c-Jun NH2-terminal kinase (JNK) or stress-activated protein kinase with multiple subisoforms. All of these signaling cascades have been shown to regulate AP-1 activity and are implicated in the control of IL-8 transcription [15]. Activation of Ras is known to result in activation of the Ras/Raf/MAPK cascade [16]. As activation of the Ras–Raf pathway increases the activity of transcription factors such as c-Jun, an important component of AP-1 [17], it seems likely that the Ras/MAPK cascade is involved in AP-1 activation. Although several studies revealed that C. difficile toxin A activates MAPK signaling in monocytes [18] and colonocytes [19, 20], the signaling pathway in intestinal epithelial cells stimulated with C. difficile toxin A has not yet been elucidated. In the present study, we investigated the role of toxin A-induced MAPK pathways in mucosal inflammation. Our results indicate that a signaling pathway involving Ras, MAPK, and AP-1 plays an important role in toxin A-induced IL-8 expression in intestinal epithelial cells and the development of enteritis in vivo.
Materials and methods
Cell culture
HT-29 human colon epithelial cells (ATCC HTB 38) were grown in DMEM (pH 7.4, Sigma Chemical, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Gibco BRL, Grand Island, NY, USA), 2 mM l-glutamine, 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), and antibiotics (100 unit/ml of penicillin and 100 μg/ml of streptomycin), as described previously [21]. Human colon epithelial cells were obtained from normal-appearing mucosa of surgically resected colons from individuals with colon cancer, as described previously [22, 23]. Freshly isolated colon epithelial cells were cultured in 2 × 106/ml in Roswell Park Memorial Institute-1640 media supplemented with 10% FBS, 2 mM l-glutamine, and antibiotics (100 unit/ml of penicillin and 100 μg/ml of streptomycin). Epithelial cell preparations contained less than 5% contamination with B cells and monocytes/macrophages, as assessed by flow cytometry using CD19/20 and CD14 as markers.
Purification of C. difficile toxin A
C. difficile toxin A was purified from a toxigenic strain (ATCC 9689) as described previously [4, 6]. Briefly, toxigenic C. difficile was cultured anaerobically at 37°C in dialysis tubing, and culture filtrates were applied to a thyroglobulin affinity column. Fractions showing cytotoxicity (assessed by the rounding of Vero cells) and hemagglutinating activity (determined using a 1% rabbit erythrocyte suspension) were subsequently subjected to two sequential anion exchange chromatographic steps with Q Sepharose FF and Mono Q columns (Pharmacia Biotech, Brussels, Belgium) incorporated into a fast protein liquid chromatography apparatus (Pharmacia Biotech). Finally, the purity of toxin A was monitored using a commercial monoclonal enzyme-linked immunosorbent assay (ELISA) kit for toxin A of C. difficile (TechLab C. difficile TOX A/B ELISA test kit, Blacksburg, VA, USA). To confirm the absence of contaminating toxin B, we performed ELISA for toxin B using the TechLab C. difficile TOX A/B ELISA test kit. In addition, cytotoxic activity against HT-29 cells was completely neutralized with specific anti-toxin A antibody. Buffers were prepared using lipopolysaccharide (LPS)-free water (Baxter Healthcare, Deerfield, IL, USA), and LPS activity in 1 mg/ml toxin A solutions was less than one endotoxin unit/ml (quantitative chromogenic limulus amebocyte lysate; BioWhittaker, Walkersville, MD, USA). Aliquots of purified toxin A at concentrations of 50 to 100 μg/ml were stored at −70°C until used.
Immunoblot analyses
Confluent monolayers in six-well plates were washed with ice-cold phosphate-buffered saline (PBS) and lysed in 0.5 ml/well lysis buffer (150 mM NaCl, 20 mM Tris, pH 7.5, 0.1% Triton X-100, 1 mM phenylmethanesulfonyl fluoride, 10 μg/ml aprotinin) as described previously [22]. Protein concentrations in the lysates were determined by the Bradford assay (Bio-Rad, Hercules, CA, USA). Fifteen to 50 μg protein/lane was size-fractionated on 6% polyacrylamide minigels (Mini-PROTEIN II; Bio-Rad) and electrophoretically transferred to a nitrocellulose membrane (0.1-μm pore size). Specific proteins were identified with polyclonal antibodies for pan-ERK1/2 (p44/p42), phospho-ERK1/2, pan-JNK (p54/p46), phospho-JNK, pan-p38, and phospho-p38 (all from Cell Signaling Technology, Bervery, MA, USA; Catalog # 9102, 9101, 9252, 9251, 9212, and 9212, respectively). The immunoreactive proteins were visualized using goat anti-rabbit secondary antibodies conjugated to horseradish peroxidase (Transduction Laboratories, Lexington, KY, USA), followed by enhanced chemiluminescence (ECL system; Amersham Life Science, Buckinghamshire, England) and exposure to X-ray film (XAR5; Eastman Kodak Company, Rochester, NY, USA).
Ras pull-down assay was performed as described according to the manufacturer’s protocol (Upstate Biotechnology, Lake Placid, NY, USA). Briefly, toxin A-stimulated cells were lysed in 2% Triton X-100, 100 mM HEPES, pH 7.5, 200 mM NaCl, 10 mM MgCl2, 2 mM sodium orthovanadate, 1:50 (v/v) mammalian cell protease inhibitor mixture, and cleared by centrifugation at 15,000×g for 2 min at 4°C. The remainder of the lysates was incubated for 90 min at 4°C with beads coated with a fusion protein (glutathione-S-transferase [GST]–Raf-1 rhotekin Rho-binding domain) consisting of GST fused to the Ras-binding domain of Raf-1 (Upstate Biotechnology). Beads were washed three times with cold PBS, 5 mM MgCl2, and 0.1% Triton X-100, and bound protein was eluted for 15 min with 2× Laemmli sample buffer that had been preheated to 95°C. The active Ras was determined by immunobloting with an ant-Ras antibody (clone RAS10, Upstate Biotechnology) according to the manufacturer’s protocol.
Reverse transcription-PCR analysis and ELISA
Total cellular RNA was extracted using an acid guanidinium–phenol–chloroform method (Trizol; Gibco BRL, Gaithersburg, MD, USA). Quantitative reverse transcription-polymerase chain reaction (RT-PCR) using an internal standard was used to quantify chemokine messenger RNA (mRNA) levels, as described previously [6]. Synthetic standard RNAs were provided by Dr. Kagnoff at the University of California, San Diego. PCR amplification consisted of 35 cycles of 1 min denaturation at 95°C and 2.5 min annealing and extension at either 60°C (IL-8) or 72°C (β-actin).
ELISA was performed in triplicate on chemokines in culture supernatants and tissue extracts. Ileal loops were obtained after toxin A challenge and homogenized with 0.3 ml PBS containing complete protease inhibitor mixture (Roche, Tokyo, Japan). Homogenates were centrifuged at 12,000×g for 15 min, and the supernatants were filtered through a 0.22-μm filter to remove any contaminants. The concentration of human IL-8 and mouse macrophage-inflammatory protein (MIP)-2 was determined using Quantikine immunoassay kits (R&D Systems, Minneapolis, MN, USA).
Electrophoretic mobility shift assay
Nuclear extracts were prepared from cells as previously described [22], and protein concentrations were determined using the Bradford assay (Bio-Rad). Electrophoretic mobility shift assay (EMSA) for AP-1 was performed according to the manufacturer’s protocol (Promega, Madison, WI, USA). In brief, 5 μg nuclear extract was incubated for 30 min at room temperature with a γ32P-labeled oligonucleotide probe (5′-CGC TTG ATG ACT CAG CCG GAA-3′) corresponding to an AP-1 binding site. After incubation, bound and free DNA was resolved on 5% native polyacrylamide gels, as described previously [22]. For competition assays, nuclear extracts were pre-incubated with AP-1 oligomer, AP-1 oligomer mutant (5′-CGC TTG ATG ACT TGG CCG GAA-3′), or NF-κB oligomer (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) for 1 h at 4°C. For supershift assays, nuclear extracts were incubated for 1 h at 4°C with rabbit antibodies (1 μg/reaction) against c-Jun, c-Fos, Jun-B, Jun-D, or Fos-B (Santa Cruz Biotechnology, Santa Cruz, CA, USA) before incubation with the radiolabeled probe.
Plasmids and transfection
TAM-67 is a dominant-negative c-Jun superrepressor that lacks the transactivation domain of c-Jun and is a potent inhibitor of AP-1-mediated transactivation [24]. TAM-67 dimerizes with c-Jun or c-Fos family members and binds DNA, resulting in the inhibition of wild-type c-Jun and c-Fos function. The TAM-67 used in the present study was a gift from Dr. Andreas von Knethen at University of Erlangen, Erlangen, Germany. N-17 is a dominant-negative Ras superrepressor containing an inhibitory Ser17Arg substitution that is present in c-Ha-ras [25]. N-17 was provided by Dr. Hyeyoung Kim at Yonsei University, Seoul, Korea [26]. As a control, pcDNA3 (Invitrogen, Carlsbad, CA, USA) was transfected into cells instead of the c-Jun or Ras mutant genes. Recombinant adenoviruses containing dominant-negative p38α (ADV-105), and dominant-negative JNK1 expression vectors were obtained from Cell Biolabs (San Diego, CA, USA). In the dominant-negative p38α plasmid, the TGY dual phosphorylation site has been changed to angioblastic growth factor, and in the dominant-negative JNK1 plasmid, the dual phosphorylation site T183/Y185 has been changed to A183/F185. The pIL8-luciferase, pβ-actin-luciferase, and pRSV-β-galactosidase transcriptional reporters were provided by Dr. Kagnoff at the University of California, San Diego [27]. The reporter plasmid containing AP-1-luciferase was purchased from BD Sciences (Franklin Lakes, NJ, USA).
Cells in six-well dishes were transfected with 1.5 μg plasmid DNA using Fugene 6 (Roche, Mannheim, Germany), according to the manufacturer’s instructions [23], and the transfected cells were incubated for 48 h at 37°C in a 5% CO2 incubator. Cells were then incubated with toxin A, harvested, and whole cell lysates were prepared as described previously [23]. Briefly, cells were lysed at 4°C for 25 min in whole cell lysis buffer (0.1 M KPO4, 0.1 M DTT, 0.5% Triton X-100, pH 7.8). Luciferase activity was determined and normalized relative to β-galactosidase expression according to with the manufacturer’s instructions (Tropix, Bedford, MA, USA). Light release was quantitated for 10 s using a luminometer (MicroLumat Plus, Berthold, Bad Wildbad, Germany). β-galactosidase activity was determined using the chemiluminescent substrate AMPGD (3-(4-methoxyspiro[1,2-dioxetane-3,2′- tricylo[3.3.1.1]decan]-4-yl)phenyl-β-d-galactopyranoside; Tropix) as described previously [23]. Light release was induced by the addition of 50 μl 0.2 N NaOH containing 10% Emerald enhancer (Tropix), and quantitated for 10 s in a luminometer. Non-transfected cells were used as a background control.
Small interfering RNA (siRNA) against c-Jun and nonsilencing siRNA were purchased from Dharmacon Research (Lafayette, CO, USA). Briefly, HT-29 cells were cultured in six-well plates to 50 to 80% confluence. The cells were then transfected with Jun-specific silencing siRNA using Fugene 6 (Roche) as transfection reagent, following the manufacturer’s instructions. Transfections were incubated for 48 h before assay.
Effect of p38 inhibitor SB203580 on C. difficile toxin A-induced enteritis in mice
Fasted (16 h)-specific pathogen-free mice (20–25 g, C57BL6Cr; Orient Experimental Animal, Kyounggi-do, Korea) were anesthetized by intraperitoneal injection of sodium pentobarbital (600 μg/mouse). Ileal loops (3–4 cm) were prepared and injected with a 200-μl buffer or p38 inhibitor SB203580-hydrochloride (100 μg in water, Sigma) as described previously [22, 23]. After 30 min, C. difficile toxin A (5 μg in PBS) was injected into the loops, and animals were killed 4 h later by pentobarbital overdose. One loop in each mouse was used in this experiment. All procedures of animal study were approved by the Animal Care Committee of Hanyang University College of Medicine. Loops were excised weighed, and the length was measured. Ileal tissue samples were fixed in formalin, paraffin embedded, and stained with hematoxylin and eosin. Tissue sections were examined by a gastrointestinal pathologist who was blinded to the study conditions and scored using the system described by Pothoulakis et al. [28] in which the severity of enteritis was graded based on the following parameters: (a) epithelial damage, (b) mucosal congestion and edema, and (c) neutrophil infiltration, with a score of 0–3, denoting increasingly severe abnormality, assigned to each parameter. To assess AP-1 activity in mouse tissue, we used a TransAM™ AP-1 family kit (Active Motif, Carlsbad, CA, USA). Briefly, nuclear proteins from tissue extracts were obtained by using the Nuclear Extract Kit (Active Motif) according to the manufacturer’s instruction. The total protein concentration of the extracts was determined by Bradford assay. The activation of c-Jun was measured using the TransAM™ AP-1 family kit (Active Motif) according to the manufacturer’s instruction in which the method measures the DNA-binding activity of AP-1 by ELISA. Specific binding was detected by colorimetric estimation at 450 nm with a reference wavelength of 655 nm. As a positive control for AP-1 activation, the K-562 (tetradecanoylphorbol-13-acetate [TPA] stimulated) nuclear extract provided with the kit was used.
Statistical analyses
Data are presented as mean±standard deviation (SD) for quantitative RT-PCR and mean±standard error of the means (SEM) for ELISA and luciferase assay. Wilcoxon’s rank sum test was used for statistical analysis. Mann–Whitney U test was used for intergroup comparisons.
Results
C. difficile toxin A activates c-Jun/c-Fos heterodimeric AP-1 DNA binding activity in human colon epithelial cells
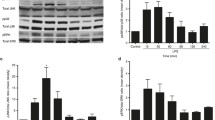
To determine whether C. difficile toxin A activates AP-1 signals in human colon epithelial cells, DNA-binding studies were performed using nuclear extracts from cells after treatment with toxin A. Stimulation of HT-29 colon epithelial cell line or primary intestinal epithelial cells with toxin A increased DNA binding activity of AP-1 (Fig. 1a and d). Binding specificity was confirmed by a competition assay with cold probes: The addition of an AP-1 oligomer to nuclear extracts after stimulation with toxin A for 1 h suppressed the AP-1 signal, whereas the addition of an AP-1 mutant oligomer or an NF-κB oligomer had no effect on binding (Fig. 1b). A supershift assay was then performed to identify the specific AP-1 subunits that comprise the AP-1 signal detected in toxin A-stimulated cells. As shown in Fig. 1c, AP-1 binding was inhibited by antibodies to c-Jun and c-Fos. However, the addition of antibodies to Jun-B, Jun-D, or Fos-B did not affect AP-1 binding activity induced by toxin A. Similar results were also observed in primary human colon epithelial cells (Fig 1e). These results indicate that toxin A can activate DNA-binding activity of AP-1, composed of c-Jun/c-Fos heterodimers.
C. difficile toxin A activates AP-1 DNA binding in colon epithelial cells. a HT-29 cell lines (above) and primary human colon epithelial cells (below) were stimulated with C. difficile toxin A (20 ng/ml) for the indicated periods of time. AP-1 DNA-binding activity was assessed by EMSA. b Competition assay for AP-1 specificity was performed by addition of each oligomer to nuclear extracts of HT-29 cells. c and d Supershift assays were performed using antibodies to c-Jun, c-Fos, Jun-B, Jun-D, and Fos-B and nuclear extracts from HT-29 cells (c) or primary human colon epithelial cells (d) stimulated with toxin A for 1 h. Results are representative of five independent experiments
Luciferase activity in the HT-29 cells transfected with AP-1 promoter plasmid correlated with the concentration of toxin A: 1 h after stimulation with 0.1, 1, 10, 50, and 100 ng/ml concentrations of toxin A, the reporter gene activity of the AP-1 construct increased 1.1 ± 0.3, 2.8 ± 0.6, 8.3 ± 0.9, 7.2 ± 1.4, and 5.4 ± 0.7-fold respectively, relative to unstimulated controls (mean value, n = 3).
Having shown that toxin A activated c-Jun/c-Fos heterodimeric AP-1 in human colon epithelial cells, we next investigated whether this AP-1 activation is associated with increased IL-8 expression. Transfection with c-Jun superrepressor completely suppressed the AP-1 DNA binding in HT-29 cells stimulated with toxin A, whereas transfection with control plasmid had no effect on binding (Fig. 2a). Treatment with toxin A also stimulated production of IL-8 (Fig. 2b). This toxin A-induced production of IL-8 was significantly decreased in cells transfected with a c-Jun superrepressor plasmid, but not in cells transfected with control plasmid (Fig. 2b). Consistent with this result, stimulation with toxin A also increased the levels of IL-8 mRNA transcript in primary colon epithelial cells, while pretreatment with the AP-1 inhibitor curcumin before toxin A stimulation resulted in a significant decrease in IL-8 mRNA expression (Table 1). To confirm the inhibition of IL-8 by AP-1 inhibitor curcumin, an experiment using siRNA against c-Jun was performed. As a result, IL-8 expression in toxin A-exposed HT-29 cells was significantly inhibited by transfection with c-Jun siRNA [control, 8.3 ± 4.2; toxin A, 196 ± 92; toxin A + c-Jun siRNA, 73 ± 190; toxin A + nonsilencing siRNA, 203 ± 107; mean no of IL-8 mRNA transcripts (×105)/μg RNA ± SD, n = 3]. In this experiment, β-actin mRNA levels in each group remained relatively constant [control, 8.6 ± 3.4; toxin A, 9.3 ± 5.0; toxin A + c-Jun siRNA, 9.8 ± 6.3; toxin A + nonsilencing siRNA, 10.6 ± 2.6; mean no of β-actin mRNA transcripts (×106) /μg RNA ± SD, n = 3].
The superrepressor of c-Jun decreased AP-1 DNA-binding activity and IL-8 secretion in HT-29 cell lines stimulated with C. difficile toxin A. HT-29 cells were transfected with a dominant-negative c-Jun expression vector (dn-c-Jun). a Forty-eight hours after transfection, cells were stimulated with C. difficile toxin A (20 ng/ml) for 1 h, and EMSA was performed. b Cells were stimulated with C. difficile toxin A for 24 h, and concentration of IL-8 protein was measured by ELISA. Data are expressed as mean±SEM (n = 7). *p < 0.05 compared with toxin A alone
C. difficile toxin A activates ERK1/2, p38, and JNK kinases in human colon epithelial cells
As MAPKs regulate cytokine production in response to a variety of stimuli [12–14], we measured the phosphorylation of MAPKs in colon epithelial cells exposed to toxin A (20 ng/ml) for various time periods. As shown in Fig. 3a, toxin A strongly induced phosphorylation of ERK1/2, p38, and JNK in HT-29 cells. All three MAPK pathways were activated within 5–10 min of stimulation, but the kinetics of activation were quite different: Levels of phospho-ERK and phospho-JNK reached their maximums after 60 and 10 min, respectively, and subsequently decreased, while phospho-p38 was induced as early as 5 min after stimulation and continued to increase for up to 2 h (Fig. 3b).
C. difficile toxin A activates MAPKs ERK1/2, p38, and JNK in human colon epithelial cells. HT-29 cell lines were stimulated with C. difficile toxin A (20 ng/ml) for the indicated periods of time. Phosphorylation of ERK1/2, p38, and JNK was measured by Western blot analysis. Results are representative of five independent experiments
MAPK can induce AP-1 activation and IL-8 expression in human colon epithelial cells stimulated with C. difficile toxin A
To further evaluate the relationship between MAPK and AP-1 activation in toxin A-stimulated epithelial cells, we examined the effect of the following kinase inhibitors: PD98059, an inhibitor of MEK1/2, a MAPK that phosphorylates ERK1/2; pyridinyl imidazole SB203580, which specifically inhibits p38; and SP600125, which inhibits JNK. As shown in Fig. 4, pretreatment of HT-29 cells with PD98059 (>50 μM), SB203580 (>10 μM), or SP600125 (>10 μM) for 30 min significantly inhibited the AP-1 transcriptional activity induced by toxin A. However, SB203580 and SP600125 had a greater inhibitory effect on AP-1 activity than PD98059 did.
Effects of MAPK inhibition on AP-1 reporter gene expression in HT-29 cells stimulated with C. difficile toxin A. HT-29 cell lines were transfected with pAP-1-luciferase transcriptional reporter. Forty-eight hours after transfection, cells were pre-incubated with each concentration of PD98059 (open circle), SB203580 (open square), or SP600125 (filled circle) for 30 min and stimulated with C. difficile toxin A (20 ng/ml) for another 1 h. Data are expressed as the mean fold induction in luciferase activity relative to unstimulated controls±SEM (n = 7). * p < 0.05 compared with toxin A alone
To confirm the role of MAPK signaling pathways in AP-1 activation by toxin A, we transfected recombinant adenoviruses into HT-29 cells and measured DNA-binding activity of AP-1. As shown in Fig. 5b, transfection with expression vectors containing dominant-negative mutants of p38 or JNK significantly decreased the activation of AP-1 after toxin A stimulation. Concurrently, transfection with adenovirus containing dominant-negative p38 or JNK expression vectors significantly inhibited IL-8 mRNA expression in toxin A-stimulated HT-29 cells. Phosphorylation of p38 or JNK was suppressed in HT-29 cells infected with the corresponding recombinant adenovirus (Fig. 5c).
Effects of transfection with recombinant adenovirus on AP-1 activation and IL-8 expression in HT-29 cell lines stimulated with C. difficile toxin A. a HT-29 cells were transfected with adenovirus containing dominant-negative p38 (dn-p38) or JNK (dn-JNK) expression vectors. Forty-eight hours after transfection, cells were stimulated with C. difficile toxin A (20 ng/ml). AP-1 DNA-binding activity was measured by EMSA 1 h after stimulation. The control means untreated HT-29 cells. b Transfected cells were stimulated with toxin A (20 ng/ml) for 6 h, and total RNA was extracted. Expression of IL-8 and β-actin mRNA was assessed by RT-PCR. Results are representative of five independent experiments. Positive and negative symbols represent positive and negative control, respectively. Total RNA extracted from HT-29 cells stimulated with TNF-α (20 ng/ml) for 8 h was used as the positive control and products obtained by PCR reaction without cDNA was used as the negative control. c Phosphorylated p38 and JNK were detected in recombinant adenovirus-transfected HT-29 cells after stimulation with toxin A (20 ng/ml) for 10 min. Results are representative of five independent experiments. 1 control; 2 toxin A alone; 3 toxin A + dominant-negative p38; 4 toxin A + dominant-negative JNK; 5 toxin A + control virus
A superrepressor for Ras inhibits AP-1 activation and IL-8 expression in C. difficile toxin A-stimulated HT-29 cells
Although Ras is known to be a major regulator of MAPK and AP-1-mediated gene transcription, its role in C. difficile toxin A stimulation remains unclear. In this study, toxin A activated the Ras signal in HT-29 cells, as determined by Ras affinity precipitation assay (Fig. 6a). To determine whether Ras is involved in the signaling pathway for AP-1 activation and IL-8 expression in toxin A-stimulated intestinal epithelial cells, we transfected HT-29 cells with a dominant-negative Ras superrepressor. As shown in Fig. 6b, transfection with the Ras superrepressor significantly reduced the induction AP-1 transcriptional activity after stimulation with toxin A, while transfection with control plasmid had no effect. In addition, activation of the IL-8 transcriptional reporter was also inhibited in cells cotransfected with a Ras superrepressor plasmid, but not in cells cotransfected with control plasmid. In this condition, transfection with the Ras superrepressor significantly reduced the levels of phosphorylated p38 and JNK in toxin A-stimulated HT-29 cells (Fig. 6c). These results suggest that the stimulation of intestinal epithelial cells with C. difficile toxin A activates a signaling cascade of Ras/MAPK/AP-1, resulting in expression of IL-8.
The Ras superrepressor inhibits AP-1 activation and IL-8 expression in HT-29 cell lines stimulated with C. difficile toxin A. a HT-29 cells were treated with C. difficile toxin A (20 ng/ml) for the indicated periods. The fraction of active Ras was determined by affinity precipitation assay. Results are representative of three independent experiments. b HT-29 cells were transfected with pAP-1 or pIL-8-luciferase transcriptional reporter together with the dominant-negative Ras superrepressor (dn-Ras) as indicated. After 48 h, the cells were stimulated with C. difficile toxin A (20 ng/ml) for 1 h (AP-1) or 6 h (IL-8), and luciferase assays were performed. Data are expressed as the mean fold induction in luciferase activity relative to unstimulated controls±SEM (n = 7). *p < 0.05 compared with toxin A alone. c Phosphorylated p38 and JNK were detected in Ras superrepressor-transfected HT-29 cells after stimulation with toxin A (20 ng/ml) for 10 min. Results are representative of five independent experiments
Inhibition of p38 attenuates C. difficile toxin A-induced enteritis in mice
The above results indicate that toxin A-induced MAPK activation is essential for AP-1 activation and IL-8 production in vitro. To assess its pathophysiological relevance in an in vivo model, we investigated whether the p38 inhibitor SB203580 would prevent toxin A-induced enteritis in mice. SB203580 was injected into the lumen of a 3- to 4-cm ileal loop (100 μg/loop) 30 min before treatment with toxin A. Administration of toxin A alone resulted in pathologic manifestations of enteritis, characterized by the destruction of villi, infiltration of neutrophils, and mucosal congestion (Fig. 7), while pretreatment with SB203580 attenuated these manifestations (Fig. 7d). Histopathological scores obtained by measuring neutrophil infiltration, congestion, and villous destruction showed that SB203580 reduced the severity of enteritis by ∼75% (Fig. 8a). In this experiment, C. difficile toxin A increased the AP-1 activity and a p38 inhibitor SB203580 decreased the AP-1 activity (toxin A, 3.9 ± 0.6; toxin A + SB203580; 1.4 ± 0.3; mean of relative values to control±SEM, n = 3), indicating that inhibition of p38 signal in vivo reduced toxin A-related AP-1 activation. In addition, the toxin A-induced secretion of MIP-2, a mouse homologue of IL-8, was significantly decreased in the SB203580-treated mice (Fig. 8b).
Inhibition of p38 MAPK reduces C. difficile toxin A-induced enteritis in murine ilea. SB203580 (100 μg/loop) was injected into the lumen of an ileal loop of specific pathogen-free C57BL6Cr mouse. After 30 min, C. difficile toxin A (5 μg/loop) was administered, and animals were sacrificed 4 h later. Results are representative of seven independent experiments. a Control; b SB203580 alone; c toxin A alone; d toxin A + SB203580. Magnifications of all figures are ×200
Inhibition of inflammation and MIP-2 production in murine ilea treated with C. difficile toxin A. The conditions of the C. difficile toxin A-treatment of murine ilea are the same as in Fig. 7. a Severity of enteritis was measured using histological scores for epithelial damage, congestion/edema, and neutrophil infiltration, as described in the “Materials and methods.” b Production of murine MIP-2 was measured by ELISA. Data represent the mean±SEM (seven loops per group). *p < 0.05 compared with C. difficile toxin A alone
Discussion
The present study demonstrated that the stimulation of intestinal epithelial cells with C. difficile toxin A resulted in the activation of AP-1 signals and IL-8 expression. The pathophysiological relevance of this association was assessed in vivo using a murine system of ileal loops. We found that the p38 inhibitor SB203580 significantly inhibited the induction of both mucosal inflammation and expression of the murine homolog of IL-8 by C. difficile toxin A, although both were significantly increased in toxin A-treated ileal loops of control mice. Together, these data indicate that MAPK signaling and AP-1 activation may be involved in inflammatory responses in a toxigenic C. difficile infection.
AP-1 functions as a transcriptional regulator by binding to the TPA response element. AP-1 is actually a collection of related transcription factors belonging to the Fos (c-Fos, Fos-B, Fra1, Fra2) and Jun (c-Jun, Jun-B, Jun-D) families, which dimerize in various combinations through a leucine zipper [29]. We demonstrated that C. difficile toxin A induced AP-1 heterodimers composed of c-Jun/c-Fos in intestinal epithelial cells. As AP-1 can be activated by various pathways, including protein kinase C, protein tyrosine kinase, and MAPK [29], we further investigated how AP-1 was activated in intestinal epithelial cells in response to toxin A and showed that C. difficile toxin A induced the phosphorylation of the MAPKs ERK1/2, p38, and JNK in intestinal epithelial cells. This finding is consistent with recent reports that toxin A can activate MAPK signals in monocytes and intestinal epithelial cells [18–20]. Moreover, inhibition of MAPK significantly decreased the activity of AP-1 and IL-8 expression. These results indicate that MAPK activation is directly associated with the induction of AP-1 transcriptional activity and IL-8 gene expression in toxin A-stimulated intestinal epithelial cells.
Ras is known to control multiple downstream effector pathways through Raf/MAPK [30, 31]. In addition, it has been reported that Ras can induce the phosphorylation and activation of transcription factors such as c-Jun [16, 32] and that c-Jun promotes chemokine expression in response to bacterial infection or enterotoxin stimulation [22, 26]. The present study showed that C. difficile toxin A activated AP-1 composed of c-Jun/c-Fos heterodimers and it is therefore likely that Ras is involved in the activation of AP-1 by toxin A. Indeed, toxin A induced Ras activation, which seem to be the most upstream member and thereby the staring point of the signaling cascade. In addition, our study showed that induction of AP-1 by toxin A was significantly inhibited by transfection with a Ras superrepressor. Furthermore, transfection with a Ras superrepressor also inhibited phosphorylation of p38 and JNK, leading to down-regulation of AP-1 activity and decreased IL-8 expression. These results clearly indicate that IL-8 expression induced by toxin A depends on the activation of Ras, a MAPK cascade and c-Jun/c-Fos heterodimeric AP-1 molecules in intestinal epithelial cells.
One of molecular mechanisms of bacterial protein toxins is glycosylation of eukaryotic target protein. In C. difficile infection, the target proteins are known to be Ras superfamily of small guanosine triphosphatase (GTPase), including Ras, Rho, Rab, Ran, and Arf [33]. For example, C. difficile toxins A and B transfer the glucose moiety of UDP-glucose to members of the Rho family of small GTPase, i.e., Rho, Rac, and Cdc42 [34]. Although the protein substrates of the C. difficile toxins A and B are found in the families of the Rho and Ras GTPase [34], it has not reported how toxin A activates Ras in intestinal epithelial cells. Therefore, further study should be needed to clarify the molecular mechanism for activating Ras molecule in toxin A-stimulated cells.
In the present study, the inhibition of AP-1 did not completely suppress IL-8 expression in vitro or the manifestation of enteritis in vivo. This partial suppression may reflect the activity of other pathways that activate NF-κB. IL-8 gene transcription requires the activation of a combination of the transcription factors NF-κB and AP-1, or NF-κB and C/EBP, depending on the type of cell or stimuli [35, 36]. We found that C/EBP was not involved in toxin A-induced IL-8 expression in HT-29 cells (as determined by EMSA, data not shown), whereas C. difficile toxin A-enhanced IL-8 transcription is dependent on NF-κB [7] and AP-1 (the present study). Furthermore, several studies demonstrated that C. difficile toxin A induced the secretion of IL-8 through MAPK [5, 18, 19] or tyrosine kinase-regulated NF-κB [7, 11]. Therefore, it is possible that both the Ras/MAPK/AP-1 pathway (in the present study) and the IκB kinase (IKK)/NF-κB pathway [7, 11], which lead to chemokine expression, may be activated in response to C. difficile toxin A stimulation. This hypothesis is consistent with the observation that Ras can activate NF-κB signaling via MAPK/extracellular signal regulated kinase kinase (MEKK). Activated MAPK/MEKK may activate IKK [37–39], which in turn mediates the phosphorylation and degradation of IκB, allowing the translocation of NF-κB to the nucleus. In addition, IKK shares structural elements with MAPK [40]. Therefore, it is possible that the two pathways are interconnected in the upstream activation pathway of AP-1.
Treatment of cultured intestinal epithelial cells with C. difficile toxin A was shown to cause mitochondrial damage, cytochrome c release, oxidative stress, and ultimately apoptosis and necrosis of those cells [4, 5, 41, 42]. In addition, activation of epithelial cell NF-κB has significant anti-apoptotic function in toxin A-treated mice [11]. Considering these reports, it is possible that early activated signal such as MAPK, NF-κB, or AP-1 may be involved in epithelial damage. Although we demonstrated that MAPK and AP-1 signaling may be associated with toxin A-induced damage, it is not clear whether this signaling pathway can regulate apoptotic processes. Recently, a study demonstrated that signaling pathway for colonocyte apoptosis after toxin A exposure involves p38-dependent activation of p53 and subsequent induction of p21 (WAF1/CIP1), resulting in cytochrome c release and caspase-3 activation through Bak induction [20]. In addition, inhibition of JNK activity reduced epithelial cell apoptosis in colitis model [43]. These findings may support a hypothesis that MPAK signals induced by toxin A stimulation may be involved in apoptotic process. Therefore, further study is needed to clarify the relationship between MAPK and apoptosis in toxin A-stimulated epithelial cells.
In summary, this study demonstrates that the exposure of intestinal epithelial cells to C. difficile toxin A results in the rapid activation of MAPK signaling pathways, leading to AP-1 activation and IL-8 gene expression. Notably, the activation of AP-1 seems to mediate intestinal inflammation and mucosal damage induced by toxin A. Based on these findings, we suggest that MAPK signaling and AP-1 activation contribute to the biological effects of toxin A produced by toxigenic C. difficile and that specific targeting of AP-1 activation may therefore be effective in the prevention or treatment of inflammation associated with infection by toxigenic C. difficile.
References
Kelly CP, LaMont JT (1998) Clostridium difficile infection. Annu Rev Med 49:375–390
Burakoff R, Zhao L, Celifarco AJ, Rose KL, Donovan V, Pothoulakis C, Percy WH (1995) Effects of purified Clostridium difficile toxin A on rabbit distal colon. Gastroenterology 109:348–354
Just I, Selzer J, von Eichel-Streiber C, Aktories K (1995) The low molecular mass GTP-binding protein Rho is affected by toxin A from Clostridium difficile. J Clin Invest 95:1026–1031
Mahida YR, Makh S, Hyde S, Gray T, Borriello SP (1996) Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut 38:337–347
He D, Sougioultzis S, Hagen S, Liu J, eates S, Keates AC, Pothoulakis C, Lamont JT (2002) Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 122:1048–1057
Kim JM, Kim JS, Jun HC, Oh YK, Song IS, Kim CY (2002) Differential expression and polarized secretion of CXC and CC chemokines by human intestinal epithelial cancer cell lines in response to Clostridium difficile toxin A. Microbiol Immunol 46:333–342
Kim JM, Lee JY, Yoon YM, Oh YK, Youn J, Kim YJ (2006) NF-kappa B activation pathway is essential for the chemokine expression in intestinal epithelial cells stimulated with Clostridium difficile toxin A. Scand J Immunol 63:453–460
Rollins BJ (1997) Chemokines. Blood 90:909–928
Roebuck KA (1999) Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-κB. Int J Mol Med 4:223–230
Jefferson KK, Smith MF Jr, Bobak DA (1999) Roles of intracellular calcium and NF-kappa B in the Clostridium difficile toxin A-induced up-regulation and secretion of IL-8 from human monocytes. J Immunol 163:5183–5191
Chae S, Eckmann L, Miyamoto Y, Pothoulakis C, Karin M, Kagnoff MF (2006) Epithelial cell IkappaB-kinase beta has an important protective role in Clostridium difficile toxin A-induced mucosal injury. J Immunol 177:1214–1220
Ip YT, Davis RJ (1998) Signal transduction by the c-Jun N-terminal kinase (JNK) from inflammation to development. Curr Opin Cell Biol 10:205–219
Karin M, Liu Z, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9:240–246
Kyriakis JM, Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81:807–869
Whitmarsh AJ, Davis RJ (1996) Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med 74:589–607
Garrington TP, Johnson GL (1999) Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 11:211–218
Binetruy B, Smeal T, Karin M (1991) Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature 351:122–127
Warny M, Keates AC, Keates S, Castagliuolo I, Zacks JK, Aboudola S, Qamar A, Pothoulakis C, LaMont JT, Kelly CP (2000) p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J Clin Invest 105:1147–1156
Chen X, Kokkotou EG, Mustafa N, Bhaskar KR, Sougioultzis S, O’Brien M, Pothoulakis C, Kelly CP (2006) Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J Biol Chem 281:24449–24454
Kim H, Kokkotou E, Na X, Rhee SH, Moyer MP, Pothoulakis C, Lamont JT (2005) Clostridium difficile toxin A-induced colonocyte apoptosis involves p53-dependent p21(WAF1/CIP1) induction via p38 mitogen-activated protein kinase. Gastroenterology 129:1875–1888
Kim JM, Eckmann L, Savidge TC, Lowe DC, Witthoeft T, Kagnoff MF (1998) Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest 102:1815–1823
Kim JM, Jung HY, Lee JY, Youn J, Lee CH, Kim KH (2005) Mitogen-activated protein kinase and activator protein-1 dependent signals are essential for Bacteroides fragilis enterotoxin-induced enteritis. Eur J Immunol 35:2648–2657
Kim JM, Lee JY, Yoon YM, Oh YK, Kang JS, Kim YJ, Kim KH (2006) Bacteroides fragilis enterotoxin induces cyclooxygenase-2 and fluid secretion in intestinal epithelial cells through NF-kappaB activation. Eur J Immunol 36:2446–2456
Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ (1993) Suppression of oncogene-induced transformation by a deletion mutant of c-Jun. Oncogene 8:877–886
Cai H, Szeberenyi J, Cooper GM (1990) Effect of a dominant inhibitory Ha-ras mutation on mitogenic signal transduction in NIH 3T3 cells. Mol Cell Biol 10:5314–5323
Seo JH, Lim JW, Kim H, Kim KH (2004) Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest 84:49–62
Elewaut D, DiDonato JA, Kim JM, Truong F, Eckmann L, Kagnoff MF (1999) NF-kappaB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol 163:1457–1466
Pothoulakis C, Castagliuolo I, LaMont JT, Jaffer A, O’Keane JC, Snider RM, Leeman SE (1994) CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci USA 91:947–951
Barnes PJ (2006) Transcription factors in airway diseases. Lab Invest 86:867–872
Boguski MS, McCormick F (1993) Proteins regulating Ras and its relatives. Nature 366:643–654
Lowy DR, Willumsen BM (1993) Function and regulation of ras. Annu Rev Biochem 62:851–891
Joneson T, Bar-Sagi D (1997) Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med 75:587–593
Wennerberg K, Rossman KL, Der CJ (2005) The Ras superfamily at a glance. J Cell Sci 118:843–846
Schirmer J, Aktories K (2004) Large clostridial cytotoxins: cellular biology of Rho/Ras-glucosylating toxins. Biochim Biophys Acta 1673:66–74
Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S (1993) Transcripton factors NF-IL-6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA 90:10193–10197
Mukaida N, Mahe Y, Matsushima K (1990) Cooperative interaction of nuclear factor κB- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by proinflammatory cytokines. J Biol Chem 265:21128–21133
Lange-Carter CA, Johnson GL (1994) Ras-dependent growth factor regulation of MEK kinase in PC12 cells. Science 265:1458–1461
Lange-Carter CA, Pleiman CM, Gardner AM, Blumer KJ, Johnson GL (1993) A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science 260:315–319
Russell M, Lange-Carter CA, Johnson GL (1995) Direct interaction between Ras and the kinase domain of mitogen-activated protein kinase kinase kinase (MEKK1). J Biol Chem 270:11757–11760
Nemoto S, DiDonato JA, Lin A (1998) Coordinate regulation of IκB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-κB inducing kinase. Mol Cell Biol 18:7336–7343
Pothoulakis C, Lamont LT (2001) Microbes and microbial toxins: paradigms for microbial-mucosal interactions. II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol 280:G178–G183
Brito GA, Fujji J, Carneiro-Filho BA, Lima AA, Obrig T, Guerrant RL (2002) Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J Infect Dis 186:1438–1447
Assi K, Pillai R, Gomez–Munoz A, Owen D, Salh B (2006) The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology 118:112–121
Acknowledgement
We thank Dr. Martin F. Kagnoff, Dr. Joseph A. DiDonato, Dr. Andreas von Knethen, and Dr. Hyeyoung Kim for several plasmids, Dr. Kyoung-Ho Kim for histolopathologic examinations, and Han-Jin Lee for the excellent technical help. This study was supported by a grant of Seoul R&BD Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.Y., Park, H.R., Oh, YK. et al. Effects of transcription factor activator protein-1 on interleukin-8 expression and enteritis in response to Clostridium difficile toxin A. J Mol Med 85, 1393–1404 (2007). https://doi.org/10.1007/s00109-007-0237-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-007-0237-7