Abstract
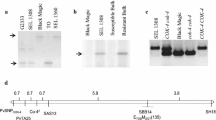
The generation of splice variants has been reported for various plant resistance (R) genes, suggesting that these variants play an important role in disease resistance. Most of the time these R genes belong to the Toll and mammalian IL-1 receptor–nucleotide-binding site–leucine-rich repeat (TIR–NBS–LRR) class of R genes. In Phaseolus vulgaris, a resistance gene cluster (referred to as the B4 R-gene cluster) has been identified at the end of linkage group B4. At this complex resistance cluster, three R specificities (Co-9, Co-y and Co-z) and two R QTLs effective against the fungal pathogen Colletotrichum lindemuthianum, the causal agent of anthracnose, have been identified. At the molecular level, four resistance gene candidates encoding putative full-length, coiled-coil (CC)–NBS–LRR R-like proteins, with LRR numbers ranging from 18 to 20, have been previously characterized. In the present study, seven cDNA corresponding to truncated R-like transcripts, belonging to the CC–NBS–LRR class of plant disease R genes, have been identified. These seven transcripts correspond to a single gene named JA1tr, which encodes, at most, only five LRRs. The seven JA1tr transcript variants result from distinct post-transcriptional modifications of JA1tr, corresponding to alternative splicing events of two introns, exon skipping and multiple ‘aberrant splicing’ events in the open reading frame (ORF). JA1tr was mapped at the B4 R-gene cluster identified in common bean. These post-transcriptional modifications of the single gene JA1tr could constitute an efficient source of diversity. The present results provide one of the few reports of transcript variants with truncated ORFs resulting from a CC–NBS–LRR gene.




Similar content being viewed by others
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res 25:3389–3402
Axtell MJ, Staskawicz BJ (2003) Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112:369–377
Ayliffe MA, Frost DV, Finnegan EJ, Lawrence GJ, Anderson PA, Ellis JG (1999) Analysis of alternative transcripts of the flax L6 rust resistance gene. Plant J 17:287–292
Bai J, Pennill LA, Ning J, Lee SW, Ramalingam J, Webb CA, Zhao B, Sun Q, Nelson JC, Leach JE, Hulbert SH (2002) Diversity in nucleotide binding site–leucine-rich repeat genes in cereals. Genome Res 12:1871–1884
Bisgrove SR, Simonich MT, Smith NM, Sattler A, Innes RW (1994) A disease resistance gene in Arabidopsis with specificity for two different pathogen avirulence genes. Plant Cell 7:927–933
Cooley MB, Pathirana S, Wu HJ, Kachroo P, Klessig DF (2000) Members of the Arabidopsis HRT/RPP8 family of resistance genes confer resistance to both viral and oomycete pathogens. Plant Cell 5:663–676
Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411:826–833
Deslandes L, Olivier J, Peeters N, Feng DX, Khounlotham M, Boucher C, Somssich I, Genin S, Marco Y (2003) Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc Natl Acad Sci USA 100:8024–8029
Dinesh-Kumar SP, Baker BJ (2000) Alternatively spliced N resistance gene transcripts: their possible role in tobacco mosaic virus resistance. Proc Natl Acad Sci USA 97:1908–1913
Dodds PN, Lawrence GJ, Ellis JG (2001) Six amino-acid changes confined to the leucine-rich repeat b-strand/b-turn motif determine the difference between the P and P2 rust resistance specificities in flax. Plant Cell 13:163–178
Ellis J, Dodds P, Pryor T (2000) Structure, function and evolution of plant disease resistance genes. Curr Opin Plant Biol 3:278–284
Ferrier Cana E, Geffroy V, Macadré C, Creusot F, Imbert-Bolloré P, Sévignac M, Langin T (2003) Characterization of expressed NBS–LRR resistance gene candidates from common bean. Theor Appl Genet 106:251–261
Flor HH (1971) Current status of the gene-for-gene concept. Annu Rev Phytopathol 9:275–296
Freyre R, Skroch P, Geffroy V, Adam-Blondon A-F, Shirmohamadali A, Johnson WC, Llca V, Nodari RO, Pereira PA, Tsai SM, Tohme J, Dron M, Nienhuis J, Vallejos CE, Gepts P (1998) Towards an integrated linkage map of common bean. 4. Development of a core linkage map and alignment of RFLP maps. Theor Appl Genet 97:847–856
Gassmann W, Hinsch ME, Staskawicz BJ (1999) The Arabidopsis RPS4 bacterial-resistance gene is a member of the TIR–NBS–LRR family of disease-resistance genes. Plant J 20:265–277
Geffroy V, Sicard D, De Oliveira JC, Sévignac M, Cohen S, Gepts P, Neema C, Langin T, Dron M (1999) Identification of an ancestral resistance gene cluster involved in the coevolution process between Phaseolus vulgaris and its fungal pathogen Colletotrichum lindemuthianum. Mol Plant–Microbe Interact 12:774–784
Geffroy V, Sévignac M, De Oliveira JCF, Fouilloux G, Skroch P, Toquet P, Gepts P, Langin T, Dron M (2000) Inheritance of partial resistance against Colletotrichum lindemuthianum in Phaseolus vulgaris and co-localization of QTL with genes involved in specific resistance. Mol Plant–Microbe Interact 13:287–296
Grant MR, Godiard L, Straube E, Ashfield T, Lewald J, Sattler A, Innes RW, Dangl JL (1995) Structure of the Arabidopsis Rpm1 gene enabling dual specificity disease resistance. Science 269:843–846
Halterman D, Zhou F, Wei F, Wise RP, Schulze-Lefert P (2001) The MLA6 coiled-coil, NBS–LRR protein confers AvrMla6-dependent resistance specificity to Blumeria graminis f. sp. hordei in barley and wheat. Plant J 25:335–348
Halterman DA, Wei FS, Wise RP (2003) Powdery mildew-induced Mla mRNAs are alternatively spliced and contain multiple upstream open reading frames. Plant Physiol 131:558–567
Hammond-Kosack KE, Jones JDG (1997) Plant disease resistance genes. Annu Rev Plant Physiol Plant Mol Biol 48:575–607
Hammond-Kosack KE, Parker JE (2003) Deciphering plant–pathogen communication: fresh perspectives for molecular resistance breeding. Curr Opin Biotechnol 14:177–193
Initiative AG (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796–815
Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19:4004–4014
Jones JDG (2001) Putting knowledge of plant disease resistance genes to work. Curr Opin Plant Biol 4:281–287
Jordan T, Schomack S, Lahaye T (2002) Alternative splicing of transcripts encoding Toll-like plant resistance proteins—what’s the functional relevance to innate immunity. Trends Plant Sci 7:392–398
Kjemtrup S, Nimchuk Z, Dangl JL (2000) Effector proteins of phytopathogenic bacteria: bifunctional signals in virulence and host recognition. Curr Opin Microbiol 3:73–78
König H, Ponta H, Herrlich P (1998) Coupling of signal transduction to alternative pre-mRNA splicing by a composite splice regulator. EMBO J 17:2904–2913
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Lawrence GJ, Finnegan EJ, Ayliffe MA, Ellis JG (1995) The L6 gene for flax rust resistance is related to the Arabidopsis bacterial resistance gene RPS2 and the tobacco viral resistance gene N. Plant Cell 7:1195–1206
Lorkovic ZJ, Wieczorek Kirk DA, Lambermon MH, Filipowicz W (2000) Pre-mRNA splicing in higher plants. Trends Plant Sci 5:160–167
Luck JE, Lawrence GJ, Dodds PN, Shepherd KW, Ellis JG (2000) Regions outside of the leucine-rich repeats of flax rust resistance proteins play a role in specificity determination. Plant Cell 12:1367–1377
Lupas A, Van Dyke M, Stock J (1991) Predicting coiled coils from protein structures. Science 252:1162–1164
Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112:379–389
Marathe R, Anandalakshmi R, Liu Y, Dinesh-Kumar SP (2002) The tobacco mosaic virus resistance gene, N. Mol Plant Pathol 3:167–172
McDowell JM, Dangl JL (2000) Signal transduction in the plant immune response. Trends Biochem Sci 25:79–82
McDowell JM, Dhandaydham M, Long TA, Aarts MGM, Goff S, Holub EB, Dangl JL (1998) Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of Arabidopsis. Plant Cell 10:1861–1874
Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CAJ (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2:253–258
Meyers BC, Chin DB, Shen KA, Sivaramakrishnan S, Lavelle DO, Zhang Z, Michelmore RW (1998) The major resistance gene cluster in lettuce is highly duplicated and spans several megabases. Plant Cell 10:1817–1832
Meyers BC, Dickerman AW, Michelmore RW, Sivaramakrihnan S, Sobral BW, Young ND (1999) Plant disease resistance genes encode members of an ancient and diverse protein family within the nucleotide-binding superfamily. Plant Cell 20:317–332
Meyers BC, Morgante M, Michelmore RW (2002) TIR-X and TIR-NBS proteins: two new families related to disease resistance TIR–NBS–LRR proteins encoded in Arabidopsis and other plant genomes. Plant J 32:77–92
Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS–LRR-encoding genes in Arabidopsis. Plant Cell 13:809–834
Modrek B, Lee C (2002) A genomic view of alternative splicing. Nat Genet 30:13–19
Nimchuk Z, Eulgem T, Holt BE, Dangl JL (2003) Recognition and response in the plant immune system. Ann Rev Genet 37:579–609
Nombela G, Williamson VM, Muniz M (2003) The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol Plant–Microbe Interact 16:645–649
Parker JE, Coleman MJ, Szabo V, Frost LN, Schmidt R, van der Biezen EA, Moores T, Dean C, Daniels MJ, Jones JDG (1997) The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the Toll and Interleukin-1 receptors with N and L6. Plant Cell 9:879–894
Parniske M, Hammond-Kosack KE, Golstein C, Thomas CM, Jones DA, Harrison K, Wulff BBH, Jones JDG (1997) Novel disease resistance specificities result from sequence exchange between tandemly repeated genes at the Cf-4/9 locus of tomato. Cell 91:821–832
Richly E, Kurth J, Leister D (2002) Mode of amplification and reorganisation of resistance genes during recent Arabidopsis thaliana evolution. Mol Biol Evol 19:76–84
Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VM (1998) The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci USA 17:9750–9754
Schornack S, Ballvora A, Gurlebeck D, Peart J, Ganal M, Baker B, Bonas U, Lahaye T (2004) The tomato resistance protein Bs4 is a predicted non-nuclear TIR-NB-LRR protein that mediates defense responses to severely truncated derivatives of AvrBs4 and overexpressed AvrBs3. Plant J 37:46–60
Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes RW (2003) Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301:1230–1233
Simpson GG, Filipowicz W (1996) Splicing of precursors to mRNA in higher plants: mechanism, regulation and sub-nuclear organisation of the spliceosomal machinery. Plant Mol Biol 32:1–41
Sinapidou E, Williams K, Nott L, Bahkt S, Tor M, Crute I, Bittner Eddy P, Beynon J (2004) Two TIR : NB : LRR genes are required to specify resistance to Peronospora parasitica isolate Cala2 in Arabidopsis. Plant J 38:898–909
Song WY, Pi LY, Wang GL, Gardner J, Holsten T, Ronald PC (1997) Evolution of the rice Xa21 disease resistance gene family. Plant Cell 9:1279–1287
Sun Q, Collins NC, Ayliffe M, Smith SM, Drake J, Pryor T, Hulbert SH (2001) Recombination between paralogues at the Rp1 rust resistance locus in maize. Genetics 158:423–438
Takahashi H, Miller J, Nozaki Y, Takeda M, Shah J, Hase S, Ikegami M, Ehara Y, Dinesh-Kumar SP (2002) RCY1, an Arabidopsis thaliana RPP8/HRT family resistance gene, conferring resistance to cucumber mosaic virus requires salicylic acid, ethylene and a novel signal transduction mechanism. Plant J 32:655–667
Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, Baker B (1994) The product of the tobacco mosaic virus resistance gene N: similarity to Toll and the Interleukin-1 receptor. Cell 78:1101–1115
Zhang XC, Gassmann W (2003) RPS4-Mediated disease resistance requires the combined presence of RPS4 transcripts with full-length and truncated open reading frames. Plant Cell 15:2333–2342
Zhu W, Schlueter SD, Brendel V (2003) Refined annotation of the Arabidopsis genome by complete expressed sequence tag mapping. Plant Physiol 132:469–484
Acknowledgements
We thank Peter Moffet and Richard Laugé for helpful discussions and critical reading of the manuscript. E.F.C. was supported by a grant from the Ministère de l’Education Nationale. The research was supported by INRA, the European community (INCO-CT980317 project), CNRS and Ministère de la recherche.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Keller
Equal contributions were made by T. Langin and V. Geffroy, who should be considered joint last authors.
Rights and permissions
About this article
Cite this article
Ferrier-Cana, E., Macadré, C., Sévignac, M. et al. Distinct post-transcriptional modifications result into seven alternative transcripts of the CC–NBS–LRR gene JA1tr of Phaseolus vulgaris . Theor Appl Genet 110, 895–905 (2005). https://doi.org/10.1007/s00122-004-1908-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-004-1908-1