Abstract
Aims/hypothesis
Defective oxidation of long-chain fatty acids is a feature of insulin resistance and Type 2 diabetes. Our aim was to compare the expression levels of the genes encoding the major proteins and enzymes of this pathway in skeletal muscle of healthy subjects and Type 2 diabetic patients.
Methods
The basal and insulin-regulated mRNA concentration of 16 genes was quantified using real-time PCR in skeletal muscle biopsies taken before and at the end of a 3-hour hyperinsulinaemic–euglycaemic clamp in healthy lean subjects and in insulin-resistant obese patients with manifest Type 2 diabetes.
Results
Acetyl CoA carboxylase-2 mRNA expression was increased 2.5-fold in the muscle of the diabetic patients. The expression of carnitine palmitoyl transferase-1, of the two adiponectin receptors and of genes involved in fatty acid transport and activation was not altered in diabetic patients. Hyperinsulinaemia for 3 hours increased the expression of several genes of fatty acid oxidation, including adiponectin receptor-1 and peroxisome proliferator-activated receptor γ coactivator-1α. It also reduced pyruvate dehydrogenase 4 mRNA levels. The effects of insulin on gene expression were markedly altered in the muscle of Type 2 diabetic patients except for adiponectin receptor-1 and pyruvate dehydrogenase 4 mRNAs.
Conclusions/interpretation
The expression of adiponectin receptors was not altered in the muscle of Type 2 diabetic patients. The observed overexpression of acetyl CoA carboxylase-2 is consistent with the hypothesis that increased skeletal muscle malonyl CoA concentrations in Type 2 diabetes may contribute to the inhibition of long-chain fatty acid oxidation.
Similar content being viewed by others
Introduction
Altered glucose and lipid metabolic pathways in skeletal muscle are major disturbances associated with insulin resistance in Type 2 diabetes mellitus [1]. In addition to decreased insulin-induced glucose uptake and utilisation, insulin resistance is characterised by a reduced ability to oxidise lipids despite elevated NEFA levels, and by an accumulation of triglycerides in skeletal muscle [1, 2]. During the postabsorptive condition, lipid oxidation is the predominant metabolic activity of resting muscles [3]. A number of studies have demonstrated an altered capacity of lipid oxidation in skeletal muscle of insulin-resistant patients, either at rest in the postabsorptive state [2], during β-adrenergic stimulation [4] or during moderate-intensity exercise [5]. In parallel to this defect, and perhaps as a consequence of it, there is a significant accumulation of triglycerides within the skeletal muscle cells of insulin-resistant subjects, which correlates negatively with insulin sensitivity [1, 6, 7]. A possible explanation for this strong association is that reduced fatty acid oxidation and excess of triglycerides can favour the intracellular accumulation of long-chain acyl CoAs (LCACoA), which leads to impaired insulin signalling in skeletal muscle, either directly or via activation of protein kinase C isoforms [1, 6, 7, 8].
To date, the cellular and molecular mechanisms responsible for the reduction in fatty acid oxidation in skeletal muscle of insulin-resistant patients are not completely understood. Mitochondrial dysfunction has recently been proposed as a possible cause. Skeletal muscle mitochondria are smaller and associated with an impaired bioenergetic capacity in Type 2 diabetic subjects [9]. A set of genes involved in oxidative phosphorylation has recently been found to be coordinately down-regulated in the skeletal muscle of Type 2 diabetic patients [10, 11]. Moreover, a strong reduction in mitochondrial oxidative and phosphorylation activities, together with intramyocellular accumulation of lipids, has been observed in the muscle of insulin-resistant subjects [12]. In addition to the mitochondria, altered content of enzymes involved in fatty acid transport and metabolism has also been reported, suggesting that the metabolic capacity of the insulin-resistant skeletal muscle is more oriented towards fat esterification than towards oxidation [4, 13]. One of the most likely candidates is a reduction in the activity of carnitine palmitoyl transferase-1 (CPT1) [6]. This enzyme regulates the entry of long-chain fatty acids (LCFA) into the mitochondria by converting LCACoA into long-chain acyl carnitine in the outer membrane. It has been suggested that decreased expression of CPT1, or a more pronounced inhibition of its activity by malonyl CoA, could explain the diminished ability to oxidise fatty acids in skeletal muscle [6].
The adipocyte-derived hormone adiponectin has been shown to promote fatty acid β-oxidation in skeletal muscle in mice [14, 15]. Moreover, the plasma adiponectin concentration is decreased in insulin-resistant subjects and correlates positively with whole-body insulin sensitivity [16], suggesting that this hormone also plays a role in the regulation of fatty acid oxidation in humans. Two receptors for adiponectin were recently cloned [17]. They are expressed in mouse skeletal muscle and their overexpression in C2C12 myotubes increased fatty acid oxidation [17]. No data are available regarding possible alterations in the expression and/or function of these receptors in skeletal muscle of insulin-resistant patients.
In this work, we compared the mRNA expression pattern of 16 genes encoding the major controlling enzyme and proteins of the fatty acid oxidative pathway (Fig. 1), including the two adiponectin receptors. This was done in muscle biopsies from insulin-sensitive healthy subjects and from obese patients with manifest Type 2 diabetes mellitus. Our data provided evidence for increased expression of acetyl CoA carboxylase (ACC2) in the skeletal muscle of Type 2 diabetic patients.
Schematic representation of the main metabolic pathway of long-chain fatty acid oxidation in skeletal muscle. The mRNA expression of the 16 proteins indicated in white boxes was quantified in the study. They included: fatty acid translocase (FAT/CD36, UniGene Cluster number: Hs.75613); plasma membrane fatty acid binding protein (FABPpm, Hs.170197); cytosolic fatty acid binding protein-3 (FABP3, Hs.49881); fatty acid CoA ligase (FACL1, Hs.406678 and FACL2, Hs.154890); carnitine palmitoyl CoA transferases (CPT1, muscle form, Hs.29331 and CPT2, Hs.274336); carnitine acyl carnitine translocase (CACT, Hs.13845); acetyl CoA carboxylase-2 (ACC2, Hs.183857); pyruvate dehydrogenase kinase 4 (PDK4, Hs.8364); adiponectin receptors (AdipoR1, Hs.5298 and AdipoR2, Hs.334854); the peroxisome proliferator-activated receptors (PPARα, Hs.998; PPARβ, Hs.106415 and PPARγ1, Hs.100724); PPARγ coactivator-1α (PGC1α, Hs.198468); and mitofusin-2 (Mfn-2, Hs.3363)
Subjects and methods
Subjects
The characteristics of the 20 subjects involved in the study are presented in Table 1. None of the control subjects had impaired glucose tolerance or a familial or personal history of diabetes, obesity, dyslipidaemia or hypertension. The Type 2 diabetic patients (HbA1c: 10.2±0.2%) interrupted their usual treatment with oral antidiabetic agents at least 5 days before the investigation (interruption done under medical control). All participants gave their written consent after being informed of the nature, purpose and possible risks of the study. The experimental protocol was approved by the Ethics Committees of the Hospices Civils de Lyon and was performed according to French legislation (Huriet law).
Study design
To characterise insulin sensitivity and to investigate the effect of insulin on target gene expression, the subjects were submitted to a 3-hour euglycaemic-hyperinsulinaemic clamp, as previously described [18, 19]. To determine metabolites, hormones and [6,6-2H2] glucose isotopic enrichment, blood samples were drawn every 10 min during the last 30 min of the basal and hyperinsulinaemic periods. Metabolite and hormone concentrations were measured using enzymatic methods and radioimmunoassays. Plasma isotopic enrichment of [6,6-2H2] glucose was determined by Gas-Chromatography-Mass Spectrometry (5971 MSD, Hewlett-Packard, Palo Alto, Calif., USA) and glucose turnover rates were calculated using steady-state equations, as previously described [18, 19]. For the diabetic patients, glucosuria was subtracted from glucose turnover rates to calculate glucose utilisation. To estimate glucose and lipid oxidation rates, respiratory exchange measurements were performed during the final 30 min of the basal and hyperinsulinaemic periods, using a flow-through canopy gas-analyser system (Deltatrac Metabolic Monitor, Datex, Helsinki, Finland) [18, 19].
Skeletal muscle biopsies and preparation of total RNA
Skeletal muscle samples were obtained by percutaneous biopsies of the vastus lateralis muscle under local anaesthesia (2% lidocaine) and using Weil Blakesley pliers, as described previously [18, 19]. The size of the biopsies averaged 60 mg with no difference between samples from control and diabetic subjects or before and after the clamp. Muscle samples were immediately frozen in liquid nitrogen and total RNA was prepared from the frozen tissue according to an established procedure [20]. Average yields of total RNA were 26±2 µg/100 mg of muscle (wet weight) and were not significantly different in tissues from control and diabetic subjects, before or after the clamp. Total RNA solutions were stored at −80 °C.
Quantification of messenger RNAs
The concentrations of the mRNAs corresponding to the genes of interest were measured by reverse transcription followed by real-time PCR using a Light-Cycler (Roche Diagnostics, Meylan, France) [21]. First-strand cDNAs were first synthesised from 500 ng of total RNA in the presence of 100 units of Superscript II (Invitrogen, Eragny, France) using both random hexamers and oligo (dT) primers (Promega, Charbonnières, France). The real-time PCR was performed in a final volume of 20 µl containing 5 µl of a 60-fold dilution of the RT reaction medium, 15 µl of reaction buffer from the FastStart DNA Master SYBR Green kit (Roche Diagnostics) and 10.5 pmol of the specific forward and reverse primers (Eurobio, Les Ulis, France). Primers were selected in order to amplify small fragments (80 to 200 bp) and to hybridise in different exons of the target sequences. A list of primers and real-time PCR conditions for each mRNA assay is available upon request (vidal@laennec.univ-lyon1.fr). For quantification, a standard curve was systematically generated with six different amounts (150 to 30,000 molecules/tube) of purified target cDNA cloned in the pGEM plasmid (Promega). Each assay was performed in duplicate and validation of the real-time PCR runs was assessed by evaluating the melting temperature of the products, and by the slope and error obtained with the standard curve. The analyses were performed using Light-Cycler software (Roche Diagnostics). The results were presented as absolute concentrations, in amol/µg of total RNA. Cyclophilin mRNA levels were measured as internal standard. A similar concentration of cyclophilin mRNA was found in skeletal muscle of control and Type 2 diabetic subjects (13.8±3.3 and 13.1±1.5 amol/µg total RNA respectively, p=0.431).
Statistical analysis
All data are presented as means ± SE. Statistical significance of the results was determined using the non-parametric Mann-Whitney test, when comparing control and Type 2 diabetic subjects. A non-parametric Wilcoxon’s test for paired values was used when comparing mRNA levels before and after the clamp in the same group. The threshold for significance was set at a p value of less than 0.05.
Results
Basal mRNA expression pattern of the target genes in skeletal muscle of control and Type 2 diabetic subjects
The lean control subjects and the obese Type 2 diabetic patients who participated in the study had classical metabolic characteristics (Table 1). In the basal state, after an overnight fast, plasma concentrations of glucose, insulin, NEFA and triglycerides were higher in the Type 2 diabetic patients than in the control subjects. Basal glucose disposal and glucose oxidation rates were similar in the two groups, and the basal lipid oxidation rate, measured by indirect calorimetry, tended to be lower in the Type 2 diabetic patients, although the difference was not significant (p=0.185). During the hyperinsulinaemic clamp, the stimulation by insulin of glucose utilisation rate was profoundly reduced in obese Type 2 diabetic patients. Both insulin-stimulated non-oxidative glucose disposal and glucose oxidation rates were markedly decreased when compared with the corresponding values in healthy lean subjects. In addition, NEFA concentrations during the clamp remained higher in the Type 2 diabetic patients (Table 1).
The basal concentrations of the 16 transcripts in vastus lateralis muscle, determined by quantitative RT-PCR, are presented in Figs. 2, 3 and 4. The mRNA levels of Glut4 and hexokinase II were measured as control in the muscle samples from the two groups. In agreement with a number of previous reports [19, 22], the Type 2 diabetic patients in this study had an approximately two-fold reduction in the mRNA abundance of hexokinase II when compared with control subjects (2.8±0.6 vs 1.6±0.2 amol/µg total RNA, control vs diabetic subjects, p=0.047), whereas Glut4 mRNA expression was not different between the groups (14±3 vs 13±3 amol/µg total RNA, control vs diabetic subjects, p=0.415).
Comparison of the mRNA levels of genes coding proteins involved in long-chain fatty acid uptake, transport and activation in the skeletal muscle of control and Type 2 diabetic subjects. Specific mRNA levels were quantified by real-time PCR in total RNA preparations from vastus lateralis skeletal muscle biopsies from healthy lean subjects (white bars) and from obese Type 2 diabetic patients (black bars). Abbreviations, see legend to Fig. 1. Data are means ± SE
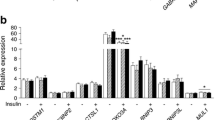
Comparison of the mRNA levels of genes coding proteins involved in the transcriptional regulation of genes of fatty acid metabolism in the skeletal muscle of control and Type 2 diabetic subjects. Specific mRNA levels were quantified by real-time PCR in total RNA preparations from vastus lateralis skeletal muscle biopsies from healthy lean subjects (white bars) and from obese Type 2 diabetic patients (black bars). *p=0.08; abbreviations, see legend to Fig. 1. Data are means ± SE
Comparison of the mRNA levels of genes coding proteins potentially involved in the regulation of fatty acid oxidation in the skeletal muscle of control and Type 2 diabetic subjects. Specific mRNA levels were quantified by real-time PCR in total RNA preparations from vastus lateralis skeletal muscle biopsies from healthy lean subjects (white bars) and from obese Type 2 diabetic patients (black bars). * p<0.05 using the non-parametric Mann-Whitney test. Abbreviations, see legend to Fig. 1. Data are means ± SE
Figure 2 shows the mRNA abundance of seven genes involved in long-chain fatty acyl CoA (LCFA) trans-membrane transport, cytosolic activation or entry into the mitochondria. The expression of fatty acid transporter protein-1 has been studied previously and was found not to differ in skeletal muscle of control and Type 2 diabetic subjects [23]. Here, we found no difference in the mRNA expression levels of the other potential membrane carriers (FAT/CD36 and FABPpm) and of FABP3, the major isoform of cytosolic fatty acid binding protein expressed in skeletal muscle. Activation of LCFAs into LCFACoAs is catalysed by fatty acid CoA ligase (FACL), also called acyl CoA synthase. Due to high sequence similarity, the RT-PCR assay did not discriminate between FACL1 and FACL2 mRNAs. There was no difference in FACL mRNA abundance in the skeletal muscle of control and Type 2 diabetic subjects (Fig. 2). LCFACoAs enter the mitochondria via the carnitine palmitoyl CoA transferase (CPT) complex (Fig. 1). This includes: (i) CPT1 in the outer membrane; (ii) carnitine acyl carnitine translocase (CACT), which mediates the transfer of acyl carnitine into the mitochondria; and (iii) CPT2, which converts long-chain acyl carnitine into LCACoA in the inner membrane of the mitochondria, where fatty acids are finally oxidised [24]. We did not find a significant difference in the basal expression levels of these three genes in control and Type 2 diabetic subjects (Fig. 2). Particularly, there was no alteration in the expression of CPT1 mRNA in skeletal muscle of diabetic subjects. Two forms of CPT1 could exist in skeletal muscle [6]. Figure 2 shows the data of the so-called muscle form, which is the most abundantly expressed in skeletal muscle. We also quantified the mRNA concentration of the liver form of CPT1 (Hs.259785), which was about 25-fold lower than that of the muscle form, with no significant difference between control and diabetic subjects (0.5±0.1 vs 0.5±0.1 amol/µg total RNA in control vs diabetic muscle, p= 0.333).
The basal mRNA levels of relevant proteins involved in transcriptional regulation are shown in Fig. 3. The nuclear receptors of the peroxisome proliferator-activated receptors (PPARs) family are involved in controlling expression of a number of genes of lipid metabolism [25]. There was no difference in the mRNA expression levels of any of the PPAR isoforms in muscle between lean control subjects and Type 2 diabetic patients (Fig. 3). PPARα was more abundantly expressed than the other PPARs, and PPARγ1 mRNA was at the limit of detection using quantitative RT-PCR. PPARγ2 mRNA was not detectable in human skeletal muscle (data not shown). We also investigated the expression of PPARγ coactivator-1α (PGC1α), a cofactor known to play a role in mitochondrial biogenesis and in regulating the expression of genes involved in oxidative phosphorylation and in glucose and fatty acid metabolism [26]. We observed an approximately 30% reduction in the mRNA levels of the cofactor PGC1α in the muscle of Type 2 diabetic patients (0.9±0.1 vs 0.6±0.1 amol/µg total RNA, control vs diabetic subjects, p=0.08), but the difference was not significant.
Figure 4 presents the results of the genes encoding possible regulators of fatty acid oxidation and mitochondrial function. The mRNA expression of the two adiponectin receptors was readily quantifiable in human skeletal muscle. AdipoR1 gene appeared to be more abundantly expressed than AdipoR2, but there was no difference in their mRNA levels between Type 2 diabetic patients and lean control subjects (Fig. 4). We also found no difference in mRNA expression of pyruvate dehydrogenase kinase 4 (PDK4) and mitofusin-2 (Mfn-2), a recently characterised protein that may participate in maintenance of the mitochondrial network [27].
The only significant difference between Type 2 diabetic patients and control subjects was observed for ACC2 mRNA (Fig. 4). Type 2 diabetic patients had a more than 2.5-fold increase in the mRNA levels of ACC2 in skeletal muscle (9±3 vs 25±5 amol/µg total RNA, control vs diabetic subjects, p=0.006).
Increased expression of ACC2 in the muscle of Type 2 diabetic patients was not associated with obesity or chronic hyperglycaemia
To verify whether the observed alteration in ACC2 expression (Fig. 4) was specific to Type 2 diabetes, we determined ACC2 mRNA levels in skeletal muscle of non-diabetic obese individuals. Biopsies were obtained from nine non-diabetic obese subjects with normal glucose tolerance and no familial history of diabetes (four men, five women, age: 47±2 years, BMI: 34.8±1.0 kg/m2, fasting glycaemia: 5.4±0.3 mmol/l, fasting insulinaemia: 11±3 mU/l). They were insulin-resistant as assessed by a 3-hour hyperinsulinaemic clamp (insulin-induced glucose disposal: 5.1±0.6 mg·kg−1·min−1, p=0.028 vs the control subjects). The hyperinsulinaemic clamps were performed under the same experimental conditions as in the other subjects (Table 1) (insulinaemia: 190±34 mU/l and glycaemia: 5.1±0.3 mmol/l). Figure 5 shows that ACC2 mRNA levels were not increased in the skeletal muscle of non-diabetic obese subjects.
Comparison of mRNA levels of acetyl CoA carboxylase-2 (ACC2) in the skeletal muscle of control subjects, non-diabetic obese individuals, and of Type 1 and Type 2 diabetic patients. ACC2 mRNA levels were determined by real-time PCR in muscle biopsies from the different groups of subjects. ACC2 mRNA expression was significantly increased in the muscle of Type 2 diabetic patients (p=0.004 using Kruskal-Wallis analysis for analysis of all groups, and p=0.006 between control subjects and Type 2 diabetic patients using the non-parametric Mann-Whitney test). Obese, non-diabetic obese subjects; T1DM, Type 1 diabetic subjects; T2DM, Type 2 diabetic subjects. Data are means ± SE
We also included a group of nine Type 1 diabetic patients (four men, five women) with similar levels of HBA1c (9.2±0.3%) to those of the Type 2 diabetic patients (10.2±0.2%). This was done to assess whether chronic hyperglycaemia could have affected the expression of ACC2 in skeletal muscle. The characteristics of these subjects have been described in detail previously [19]. They were not insulin-resistant as estimated by the mean of a 3-hour hyperinsulinaemic clamp (insulin-induced glucose disposal: 8.6±0.8 mg·kg–1·min–1, p=0.45 vs control subjects). As shown in Fig. 5, the Type 1 diabetic patients had normal expression levels of ACC2 in skeletal muscle.
Importantly, normalisation of the ACC2 mRNA data by the expression levels of a housekeeping gene (cyclophilin) did not modify the results (control subjects: 66.3±14.8% of cyclophilin; non-diabetic obese subjects: 71.3±19% of cyclophilin, p=0.42 vs control subjects; Type 1 diabetic patients: 40.4±6.9% of cyclophilin, p=0.09 vs control subjects; Type 2 diabetic patients: 186.4±27.4% of cyclophilin, p=0.0003 vs control subjects).
Effects of 3-hour insulin infusion on mRNA expression
To investigate the effect of insulin on the expression of the genes involved in fatty acid oxidation and to verify whether such regulation is altered in muscle of Type 2 diabetic patients, mRNA levels of the 16 target genes were determined in muscle biopsies taken at the end of a 3-hour hyperinsulinaemic–euglycaemic clamp in six subjects per group. The levels of FAPBpm, CPT1, CPT2, CACT, Mfn-2, ACC2, AdipoR2 and PPARα mRNAs were not modified during the clamp in either group (data not shown). Figure 6 shows the mRNA levels of the other genes before and after the clamp. Hyperinsulinaemia increased the mRNA expression of FAT/CD36, FABP3, FACL, PGC1α and PPARγ in the skeletal muscle of lean control subjects. This up-regulation was completely blunted in the muscle of Type 2 diabetic patients (Fig. 6). Analysis (by non-parametric Mann-Whitney test) of the difference in variation of the mRNA levels of these genes during the clamp showed that the difference between control and diabetic subjects was significant for PGC1α (p=0.033) and PPARγ (p=0.004). AdipoR1 mRNA expression was increased during the clamp in control subjects and in Type 2 diabetic patients (increase about 2-fold in both groups, p=0.041 and p=0.027 in control and diabetic subjects respectively). Finally, the mRNA levels of PDK4 were decreased after 3 hours of insulin infusion (Fig. 6), and as for AdipoR1, the regulation of PDK4 expression was not affected by insulin resistance and Type 2 diabetes (about 50% decrease in both groups, p=0.015 and p=0.033 in control and diabetic subjects respectively).
Effects of insulin infusion on the mRNA levels of target genes in the skeletal muscle of control and Type 2 diabetic subjects. The mRNA levels were measured in skeletal muscle biopsies taken from six healthy lean subjects (top line of figure) and six Type 2 diabetic patients (lower line) before (white bars) and after (hatched bars) a 3-hour hyperinsulinaemic–euglycaemic clamp. * p<0.05 for after vs before 3-h insulin infusion (using Wilcoxon’s non-parametric test for paired values). Abbreviations, see legend to Fig. 1. Data are means ± SE
Discussion
We have demonstrated a significant increase in the basal expression level of ACC2 mRNA in the skeletal muscle of Type 2 diabetic patients when compared with that in insulin-sensitive lean control subjects. This alteration was not found in the skeletal muscle of non-diabetic obese individuals and Type 1 diabetic patients, indicating that it was not related to obesity-associated insulin resistance or chronic hyperglycaemia. This more than 2.5-fold increase in ACC2 mRNA levels was the only perturbation found among the 16 different genes involved in fatty oxidation and measured in this study. Specifically, we did not find any difference between control and diabetic subjects in the mRNA levels of CPT1 (muscle and liver forms), the rate-limiting enzyme of LCFA catabolism into the mitochondria. If the observed increase in the mRNA concentration of ACC2 results in a higher protein content and activity, the formation of malonyl CoA could be enhanced in the skeletal muscle of Type 2 diabetic patients. In agreement with this hypothesis, a recent report strongly suggested that there is a higher rate of malonyl CoA synthesis in the skeletal muscle of subjects with Type 2 diabetes [28]. Moreover, it has been shown that the malonyl CoA fuel-sensing mechanism can operate in humans [29, 30].
Taken together, these data support the hypothesis of malonyl CoA/CPT1 interaction [6], which suggests that a higher rate of malonyl CoA production in the muscle, leading to inhibition of CPT1, reduces fatty acid oxidation and promotes re-orientation of the metabolic fate of fatty acids toward esterification and storage within the muscle cells in insulin-resistant Type 2 diabetic patients. Measurement of the activity of ACC2 and study of its regulation in skeletal muscle of diabetic subjects could provide more support for this hypothesis.
Adiponectin (also called Acrp30) is a circulating protein produced almost exclusively by adipose tissue. The concentration of adiponectin correlates with whole-body insulin sensitivity [16]. Experiments in rodents have demonstrated that adiponectin plays a role in regulating fatty acid metabolism in skeletal muscle. Administration of adiponectin to mice increased fatty acid oxidation [14, 15] and induced the expression of several genes involved in this pathway (FAT/CD36, PPARα, acyl CoA oxidase) [31]. Furthermore, incubation of rat muscle with recombinant adiponectin resulted in a significant reduction of ACC activity and consequently in a decrease in malonyl CoA concentrations [15].
The receptors for adiponectin were cloned very recently and two forms, generated by distinct genes, were found to be expressed in mice skeletal muscle [17]. We confirmed here the presence of these two receptors in human muscle, with AdipoR1 mRNA being more abundant than AdipoR2 both in lean control and in insulin-resistant subjects. There was no alteration in the expression of adiponectin receptors in the skeletal muscle of Type 2 diabetic patients. However, defective signalling of these receptors in muscle cells of insulin-resistant individuals cannot be excluded at this stage of the study and remains to be investigated.
Using microarray analysis of human muscle transcriptome, two groups recently reported that the expression of a set of genes involved in oxidative phosphorylation was decreased in Type 2 diabetic patients [10, 11]. These genes appeared to be co-regulated by PCG1α-activated transcription factors and the expression of this cofactor was found to be reduced in the muscle of Type 2 diabetic patients. These data led the authors to suggest that decreased expression of PGC1α may be involved in the disturbances of oxidative metabolism in insulin resistance and Type 2 diabetes [10, 11]. We found that the mRNA levels of PGC1α in the muscle of the obese diabetic patients were lower than those in muscle of lean control subjects. Although the difference was not significant, the magnitude (about 30%) was in the same range as previously reported [10, 11]. Direct investigation of the function of PGC1α in human muscle and its defects in insulin resistance should increase understanding of the possible role and importance of this cofactor in the pathogenesis of Type 2 diabetes.
In addition to the comparison of mRNA levels in the basal state, our study provided new data regarding in vivo regulation of the expression of the genes involved in fatty acid metabolism in human skeletal muscle. A 3-hour period of hyperinsulinaemia was associated with significant increases in the mRNA levels of FAT/CD36, FABP3, FACL and PPARγ in lean control subjects. All these genes code for proteins implicated in the entry and storage of fatty acids. This was consistent with the metabolic action of insulin, which has consistently been shown to increase uptake of fatty acids, particularly through FAT/CD36 [32], and to stimulate their esterification in vivo and in vitro in muscle [33]. Insulin infusion also induced the mRNA expression of PGC1α in the muscle of lean control subjects. Interestingly, it was recently shown that insulin stimulates mitochondrial oxidative phosphorylation [34] and up-regulates expression of the set of genes coding the enzymes of this pathway [21, 34] in human skeletal muscle in vivo. Changes in PGC1α expression during the hyperinsulinaemic clamp suggest that PGC1α could participate in this coordinated transcriptional action of insulin on this set of genes.
The mRNA expression of AdipoR1, but not of ApidoR2, was significantly induced during the 3 hours of hyperinsulinaemia. Several reports have shown an effect of adiponectin on glucose transport and metabolism [15, 16]. Moreover, overexpression of AdipoR1 in cultured muscle cells increased glucose transport [17]. The induction of AdipoR1 by insulin during the clamp could thus be consistent with a role of this receptor in pathways other than fatty acid oxidation.
Pyruvate dehydrogenase kinase 4 is an important regulator of pyruvate dehydrogenase, the rate-limiting enzyme of pyruvate, and thus glucose oxidation [35]. PDK4 phosphorylates pyruvate dehydrogenase, leading to reduced pyruvate oxidation and enhanced use of fatty acids as energy fuel by the mitochondria [35]. We found a decreased expression of PDK4 during hyperinsulinaemia. This effect was consistent with the stimulation of glucose oxidation observed during the clamp.
The induction of FAT/CD36, FABP3, FACL, PPARγ and PGC1α mRNAs during the hyperinsulinaemic clamp was prevented in the obese Type 2 diabetic patients. A substantial body of data has shown that the regulation of gene expression by insulin is altered in the skeletal muscle of insulin-resistant subjects [19, 36, 37]. In the present study, however, only a small number of subjects (n=6 per group) participated in the investigation of insulin action on gene expression. Differential regulation during the clamp appeared to be significant only for PPARγ and PGC1α mRNAs, strengthening the possible role of a deregulation at the level of the regulation of transcription in Type 2 diabetes [10, 11, 19]. Interestingly, however, the regulation of AdipoR1 and PDK4 expression was not altered in the muscle of diabetic patients. This suggested that different mechanisms of action of insulin on gene expression could occur. Some of these mechanisms could be altered in diabetic patients, whereas others could be independent of the disease. Alternatively, effects on the stability and turn-over of specific mRNAs during the clamp could also contribute to the differential regulation. Finally, it is important to note that several parameters varied during the clamp, and it cannot therefore be ruled out that some effects on gene expression were not directly dependent on insulin action.
In summary, we compared the pattern of mRNA expression of 16 genes coding proteins of fatty acid oxidative metabolism in skeletal muscle of lean control subjects and Type 2 diabetic patients. We found that both adiponectin receptors are expressed in human skeletal muscle and that there is no defect in their mRNA expression levels in Type 2 diabetes. A marked increased in the basal mRNA levels of ACC2 without alteration in CPT1 expression was observed in the muscle of Type 2 diabetic patients, supporting the hypothesis that the malonyl CoA/CPT1 interaction might play a crucial role in the reduction of fatty acid oxidation seen in the muscle of diabetic patients.
Abbreviations
- ACC2:
-
acetyl CoA carboxylase-2
- AdipoR1:
-
adiponectin receptor 1
- AdipoR2:
-
adiponectin receptor 2
- CACT:
-
carnitine acyl carnitine translocase
- CPT1:
-
carnitine palmitoyl CoA transferase 1
- CPT2:
-
carnitine palmitoyl CoA transferase 2
- FABP3:
-
fatty acid binding protein 3
- FABPpm:
-
plasma membrane fatty acid binding protein
- FACL:
-
fatty acid CoA ligase
- FAT/CD36:
-
fatty acid translocase
- LCACoA:
-
long-chain acyl CoAs
- LCFA:
-
long-chain fatty acid
- LCFACoA:
-
long-chain fatty acid coenzyme A
- Mfn-2:
-
mitofusin-2
- PDK4:
-
pyruvate dehydrogenase kinase 4
- PGC1α:
-
PPARγ coactivator-1α
- PPARs:
-
peroxisome proliferator-activated receptors
References
Kelley DE, Mandarino LJ (2000) Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49:677–683
Kelley DE, Simoneau JA (1994) Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94:2349–2356
Dagenais GR, Tancredi RG, Zierler KL (1976) Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest 58:421–431
Blaak EE, Wagenmakers AJ, Glatz JF et al. (2000) Plasma FFA utilization and fatty acid-binding protein content are diminished in Type 2 diabetic muscle. Am J Physiol Endocrinol Metab 279:E146–E154
Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA (2000) Impaired oxidation of plasma-derived fatty acids in Type 2 diabetic subjects during moderate-intensity exercise. Diabetes 49:2102–2107
McGarry JD (2002) Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of Type 2 diabetes. Diabetes 51:7–18
Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW (2003) The role of intramuscular lipid in insulin resistance. Acta Physiol Scand 178:373–383
Shulman GI (2000) Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176
Kelley DE, He J, Menshikova EV, Ritov VB (2002) Dysfunction of mitochondria in human skeletal muscle in Type 2 diabetes. Diabetes 51:2944–2950
Patti ME, Butte AJ, Crunkhorn S et al. (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471
Mootha VK, Lindgren CM, Eriksson KF et al. (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273
Petersen KF, Befroy D, Dufour S et al. (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142
Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE (1999) Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 13:2051–2060
Fruebis J, Tsao TS, Javorschi S et al. (2001) Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98:2005–2010
Tomas E, Tsao TS, Saha AK et al. (2002) Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99:16309–16313
Berg AH, Combs TP, Scherer PE (2002) ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab 13:84–89
Yamauchi T, Kamon J, Ito Y et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769
Laville M, Auboeuf D, Khalfallah Y, Vega N, Riou JP, Vidal H (1996) Acute regulation by insulin of phosphatidylinositol-3-kinase, Rad, Glut 4, and lipoprotein lipase mRNA levels in human muscle. J Clin Invest 98:43–49
Ducluzeau PH, Perretti N, Laville M et al. (2001) Regulation by insulin of gene expression in human skeletal muscle and adipose tissue. Evidence for specific defects in Type 2 diabetes. Diabetes 50:1134–1142
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Rome S, Clement K, Rabasa-Lhoret R et al. (2003) Microarray profiling of human skeletal muscle reveals that insulin regulates approximately 800 genes during a hyperinsulinemic clamp. J Biol Chem 278:18063–18068
Vestergaard H, Bjorbaek C, Hansen T, Larsen FS, Granner DK, Pedersen O (1995) Impaired activity and gene expression of hexokinase II in muscle from non-insulin-dependent diabetes mellitus patients. J Clin Invest 96:2639–2645
Binnert C, Koistinen HA, Martin G et al. (2000) Fatty acid transport protein-1 mRNA expression in skeletal muscle and in adipose tissue in humans. Am J Physiol Endocrinol Metab 279:E1072–E1079
Kerner J, Hoppel C (2000) Fatty acid import into mitochondria. Biochim Biophys Acta 1486:1–17
Lee CH, Olson P, Evans RM (2003) Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 144:2201–2207
Knutti D, Kralli A (2001) PGC-1, a versatile coactivator. Trends Endocrinol Metab 12:360–365
Bach D, Pich S, Soriano FX et al. (2003) Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 278:17190–17197
Bavenholm PN, Kuhl J, Pigon J, Saha AK, Ruderman NB, Efendic S (2003) Insulin resistance in Type 2 diabetes: association with truncal obesity, impaired fitness, and atypical malonyl coenzyme A regulation. J Clin Endocrinol Metab 88:82–87
Bavenholm PN, Pigon J, Saha AK, Ruderman NB, Efendic S (2000) Fatty acid oxidation and the regulation of malonyl-CoA in human muscle. Diabetes 49:1078–1083
Rasmussen BB, Holmback UC, Volpi E, Morio-Liondore B, Paddon-Jones D, Wolfe RR (2002) Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase-1 activity and fat oxidation in human skeletal muscle. J Clin Invest 110:1687–1693
Yamauchi T, Kamon J, Waki H et al. (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946
Glatz JF, Bonen A, Luiken JJ (2002) Exercise and insulin increase muscle fatty acid uptake by recruiting putative fatty acid transporters to the sarcolemma. Curr Opin Clin Nutr Metab Care 5:365–370
Dyck DJ, Steinberg G, Bonen A (2001) Insulin increases FA uptake and esterification but reduces lipid utilization in isolated contracting muscle. Am J Physiol Endocrinol Metab 281:E600–E607
Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS (2003) Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100:7996–8001
Sugden MC, Bulmer K, Holness MJ (2001) Fuel-sensing mechanisms integrating lipid and carbohydrate utilization. Biochem Soc Trans 29:272–278
Andersen PH, Lund S, Vestergaard H, Junker S, Kahn BB, Pedersen O (1993) Expression of the major insulin regulatable glucose transporter (GLUT4) in skeletal muscle of noninsulin-dependent diabetic patients and healthy subjects before and after insulin infusion. J Clin Endocrinol Metab 77:27–32
Pendergrass M, Koval J, Vogt C et al. (1998) Insulin-induced hexokinase II expression is reduced in obesity and NIDDM. Diabetes 47:387–389
Acknowledgements
This work was supported in part by grants from INSERM, INRA, Institut de Recherche Servier and a research grant from ALFEDIAM-Roche. The authors acknowledge Drs R. Rabasa-Lhoret and P-H. Ducluzeau for performing part of the clamp study presented in this work, and Dr A. Zorzano for providing Mfn2 cDNA. We would also like to thank C. Urbain, J. Peyrat and M. Odeon for excellent technical assistance and Professor J.-P. Riou for keen interest and helpful discussions. Cyrille Debard is the recipient of a doctoral fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche, France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Debard, C., Laville, M., Berbe, V. et al. Expression of key genes of fatty acid oxidation, including adiponectin receptors, in skeletal muscle of Type 2 diabetic patients. Diabetologia 47, 917–925 (2004). https://doi.org/10.1007/s00125-004-1394-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1394-7