Abstract
Aims/hypothesis
NEFA play a key role in the setting of insulin resistance and hyperinsulinaemia, which are both features of the prediabetic state. In addition to the direct effects on pancreas and peripheral tissues, NEFA have been reported to act via changes in autonomic nervous system activity. The present study was aimed at studying the effects of a local increase in NEFA in the brain on glucose-induced insulin secretion (GIIS) and on insulin action. We hypothesised that cerebral NEFA beta oxidation is a prerequisite for these central effects.
Methods
Male Wistar rats were infused with Intralipid/heparin for 24 h through the carotid artery towards the brain (IL rats), after which we performed the GIIS test, a euglycaemic–hyperinsulinaemic clamp and c-fos immunochemistry. In another series of experiments, Intralipid/heparin infusion was coupled with lateral ventricular infusion of etomoxir, a CPT1 inhibitor, which was initiated 5 days previously.
Results
During the infusion period, there were no changes in plasma NEFA, insulin or glucose concentrations. IL rats displayed an increased GIIS compared with control rats (C rats) infused with saline/heparin, and their liver insulin sensitivity was decreased. Furthermore, lipid infusion induced a significant decrease in c-fos-like immunoreactive neurons in medial hypothalamic nuclei, and an increase in lateral hypothalamus. Neuronal activation profile was almost normalised in IL rats infused with etomoxir, and GIIS was strongly decreased, possibly because of the concomitant normalisation of hepatic glucose output.
Conclusions/interpretation
These results strongly suggest that beta oxidation is required for the central effects of NEFA on GIIS.
Similar content being viewed by others
Introduction
Among the many aspects of type 2 diabetes in obese subjects that remain unclear, the mechanisms contributing to the early stages of the dysregulation of insulin secretion and action require further investigation [1]. Insulin hypersecretion in response to glucose is a risk factor for obesity and type 2 diabetes in predisposed patients. Dyslipidaemia, and particularly increased plasma NEFA concentrations and their adverse effects on autonomic nervous system function, could be a major contributing factor for this situation [2]. We have previously shown that an increase in plasma NEFA leads to a decrease in sympathetic nervous activity, which, in turn, has a role to play in exaggerated glucose-induced insulin secretion (GIIS) [3]. Some of the effects of NEFA appeared to be centrally mediated, since intracerebroventricular (ICV) infusion of triglycerides [4] or oleic acid [5] altered hepatic glucose output [4] as well as food intake [5]. Furthermore, ICV infusion of lipids was associated with decreased sympathetic output to the pancreas and liver [4]. Recently, NEFA have been shown to act on neurons of the arcuate nucleus of the hypothalamus [6, 7]. Thus, just like glucose, circulating NEFA appeared to be monitored by hypothalamic areas playing a critical role in the control of energy homeostasis. However, the molecular mechanisms by which NEFA exert their control of neuronal activity remain unclear. A recent study suggested that changes in the rate of beta oxidation in specific hypothalamic neurons could modulate food intake and glucose production [8].
The present study aimed to test the hypothesis that an increase in beta oxidation caused by uptake of NEFA in the brain could lead to two early major events of prediabetic state, an increase in GIIS and a decrease in insulin sensitivity. To that end, a triglyceride emulsion was infused into male Wistar rats through the carotid artery for 24 h. This physiological approach allowed NEFA levels in the brain to be increased without bypassing the blood–brain barrier. To assess the role of beta oxidation with respect to the effects of central NEFA, rats were infused intracerebroventricularly with etomoxir, an inhibitor of CPT1, before and during intracarotid infusion of NEFA.
Materials and methods
Experimental animals
The experimental protocol was approved by the institutional Animal Care and Use Committee of the University of Paris 7. Two-month-old rats (225–250 g) were used (Charles River, l’Arbresle, France). They were housed individually in stainless steel cages in a room maintained at 24±3 °C with lights on from 07.00 hours to 19.00 hours. They were fed on a laboratory diet (proteins 19.4%; carbohydrates 59.5%; lipids 4.6%; vitamins and minerals 16.5%) and water ad libitum.
Cerebral lipid infusions
The long-term infusion technique under unrestrained conditions was used, as previously described [9]. Briefly, 5 days before the beginning of the infusion, rats were anaesthetised with pentobarbital (50 mg/kg i.p.; Sanofi, Libourne, France) for the insertion of a catheter in the carotid artery towards the brain. Then, the catheter was exteriorised at the vertex of the head and attached to a swivelling infusion device, allowing the animal free access to water and food. Rats infused with a triglyceride emulsion (Intralipid 20%; KabeVitrum, Stockholm, Sweden) plus heparin (20 U/ml) were named IL rats, whereas control rats (C rats) were infused with saline/heparin. The rate of infusion was 2 µl/h. Blood was removed once daily (~80 µl) from caudal vessels for measurement of plasma NEFA, glucose and insulin concentrations.
Intracerebroventricular infusion of etomoxir
In another series of experiments, ICV infusion of etomoxir was performed in IL rats (IL ETO rats) and C rats (C ETO rats), starting 5 days before and continuing through the 24 h of intracarotid infusion. Rats were stereotaxically implanted with a chronic stainless steel cannula in the right lateral cerebral ventricle using the following coordinates from Bregma: anterior–posterior −0.8 mm; dorsal–ventral −3.5 mm; and medial–lateral −1.5 mm. The cannula was connected via a polyethylene catheter to a subcutaneously implanted osmotic minipump (model 2001; Alza Corporation, Palo Alto, Calif., USA). Minipumps were filled with either etomoxir (100 µmol/l) dissolved in saline plus 40% beta cyclodextrin to facilitate solubility, or saline/beta cyclodextrin (vehicle). The cannula was fixed to the skull using cyanoacrylate (Cyanolyt; Eleco Produits, Clichy, France). Infusions were initiated 4 h after surgery and were continued throughout the study. The rate of infusion was 0.5 µl/h.
Glucose-induced insulin secretion
Insulin secretion in response to glucose was investigated after 24 h of intracarotid Intralipid/heparin infusion, with or without ICV etomoxir. A single dose of glucose was injected under anaesthesia into the saphenous vein (0.5 g/kg body weight) of rats deprived of food for 4 h. Blood samples were drawn from caudal vessels at 0, 1, 3, 5, 10, 15, 20 and 30 min following glucose injection. Glycaemia was immediately measured using a glucose analyser (Roche Diagnostics, Meylan, France). Plasma was then removed and stored at −20 °C until radioimmunoassay of insulin.
Food intake
Daily food intake was measured by weighing the pellets between 09.00 hours and 10.00 hours.
Glucose turnover rate
The protocol for the measurement of glucose turnover rate, in the basal state and during euglycaemic–hyperinsulinaemic clamp, as well as the analytical methods have been previously described in detail [4]. Insulin (0.4 U·kg−1·h−1) was infused during clamp at a constant rate of 20 µl/min, following a priming dose of 20 mU.
C-fos immunochemistry
Rats were anaesthetised with pentobarbital 24 h after the beginning of lipid infusion and transcardially infused with ice-cold 0.9% saline for 10 min followed by a 20-min infusion of 4% paraformaldehyde in PBS. Brains were removed and post-fixed in ice-cold 4% paraformaldehyde for 2 h, after which they were cryoprotected in 30% sucrose in PBS for 2 to 3 days at 4 °C. They were then frozen and cut into 40-µm coronal sections on a freezing cryostat. Sections were placed on gel-coated slides, rinsed in PBS and exposed to 0.3% hydrogen peroxide for 30 min. They were then preincubated in PBS containing 3% normal goat serum and 0.25% Triton (blocking solution) for 2 h and incubated for 48 h with rabbit polyclonal anti-c-fos serum (1:20,000 dilution; Ab-5; Oncogene Sciences, San Diego, Calif., USA) in blocking solution. Subsequently, sections were incubated with biotinylated goat anti-rabbit IgG diluted at 1:600 (Vector Laboratories, Burlingame, Calif., USA) for 1 h and with streptavidin horseradish peroxidase for 30 min, both in blocking solution. C-fos expression was visualised for fos-like immunoreactivity (FLI) using diaminobenzidine and hydrogen peroxide in distilled water. Several PBS rinses were carried out between the above steps, except between blocking and incubation with primary antibody. Sections were then transferred onto microscope slides, dried, dehydrated and coverslipped.
Counting the c-fos-like immunoreactive neurons
A BH2 microscope (Olympus, Melville, N.Y., USA) connected via a colour video camera to a PC with Imagenia 2000 software (Biocom, Les Ullis, France) was used for cell counting. Each stained section was screened for visualisation of FLI in neurons. In regions of interest, fos-positive nuclei were counted following the area outlined, on numerous sections and on both sides, according to a stereotaxic atlas of the rat brain [10]. Each value represents an average number of FLI neurons in a given area.
Statistical analysis
Statistical analyses were performed using a two-factor repeated measures ANOVA. Comparisons between groups were carried out using a non-paired Student’s t test. A p value of less than 0.05 was considered statistically significant.
Results
Plasma parameters
Neither plasma insulin nor glucose concentrations were modified in IL rats compared with in controls during the infusion period. Systemic plasma NEFA concentration was not modified by infusion of lipid/heparin solution. In both groups, ICV infusion of etomoxir before and during 24 h of carotid infusion had no effect on plasma insulin, glucose or NEFA concentrations (Table 1).
Food intake
IL/heparin infusion had no effect on body weight or food intake, but ICV infusion of etomoxir significantly decreased food intake to a similar extent in control and IL rats without affecting body weight (Table 1).
C-fos immunochemistry
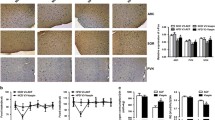
In rats infused with IL/heparin, the number of FLI neurons was found to be significantly decreased in four of the five hypothalamic nuclei studied: arcuate nucleus (ARC), dorsomedial hypothalamus (DMH), ventromedial hypothalamus (VMH) and paraventricular nucleus (PVN). In contrast, the number of FLI neurons in lateral hypothalamus (LH) was increased in IL rats (Figs. 1 and 2). The concomitant infusion of etomoxir in IL rats restored the normal neuronal activation in ARC, LH, PVN and VMH, but had no effect on DMH (Fig. 1d–f). In control rats, ICV infusion of etomoxir had no effect on the nuclei observed, except in VMH where a small but significant increase was evident (data not shown).
Photomicrographs showing c-fos-like immunoreactive neurons in five hypothalamic nuclei of C rats (left), IL rats (middle) and IL ETO rats (right). a, b and c represent ARC; d, e and f represent DM hypothalamus; g, h and i represent LH; j, k and l represent the PVN; and m, n and o represent the VMH. Magnification ×120. 3V, third ventricle
Glucose-induced insulin secretion
The time course associated with glycaemia after glucose loading was similar after 24 h of IL/heparin infusion and after saline/heparin infusion in the absence or presence of etomoxir (Fig. 3). In contrast, the glucose-induced increase in plasma insulin concentration was greater in IL rats than in C rats (Fig. 3b). In the presence of etomoxir, IL ETO rats displayed a normalisation of the GIIS, whereas etomoxir had no effect on GIIS in C ETO rats.
Time course of plasma glucose (a) and insulin (b) concentrations in response to glucose load in C rats (full squares), C ETO rats (empty squares), IL rats (full triangles, dotted line) and IL ETO rats (empty triangles, dotted line). ICV infusion (etomoxir 100 nmol/l or solvent saline/beta cyclodextrine) lasted for 6 days, and infusion through the carotid artery towards the brain lasted for 24 h. Values are means ± SEM of eight cases in each group. ** p<0.01, * p<0.05 vs controls
Glucose turnover rate
As shown in Figure 4, neither IL/heparin infusion nor ICV etomoxir administration affected GTR in the basal state. During hyperinsulinaemic clamp, plasma insulin concentrations were increased to a similar extent (~6-fold above basal values, from ~250 pmol/l to ~1500 pmol/l) in all four groups. The decrease in HGP was attenuated in IL rats compared with in control rats infused with saline/heparin (p<0.01). Intracerebral administration of etomoxir had no effect on HGP in C ETO rats but strongly decreased hepatic glucose output in IL ETO rats (p<0.001), thus restoring hepatic insulin efficiency. In contrast, the insulin-induced increase in glucose uptake was similar in all four groups.
Glucose turnover rate in the basal state (open bars) and during euglycaemic–hyperinsulinaemic clamp (solid bars) in control rats treated with placebo or etomoxir (respectively C and C ETO rats) and in IL rats treated with placebo or etomoxir (respectively IL and IL ETO rats). a. Glucose utilisation rate (GUR). b. Hepatic glucose production (HGP). Values are means ± SEM of eight cases in each group. * p<0.01 IL vs C rats; § p<0.001 IL ETO vs IL rats
Discussion
We showed that intracarotid infusion of NEFA into the brain for 24 h increased GIIS and decreased hepatic insulin sensitivity, and that these effects were blocked by the inhibition of brain CPT1 by etomoxir infusion. In addition, increasing brain NEFA concentrations for 24 h inhibited baseline c-fos expression in medial hypothalamic areas but increased expression in the lateral hypothalamus, all areas involved in the regulation of energy homeostasis. Such changes could, in part, explain exaggerated GIIS and decreased liver insulin sensitivity induced by increasing brain NEFA levels. These results are consistent with the concept of central lipotoxicity, which postulates that high levels of central lipid exposure contribute to the aetiology of type 2 diabetes [11, 12]. We propose that NEFA produced by hydrolysis of triglycerides is the main candidate in terms of exerting an effect in central areas controlling insulin secretion, as well as its action on glucose metabolism in the liver.
Since there was no increase in plasma NEFA concentration, dysregulation of insulin secretion and insulin action in IL/heparin-infused rats is probably mediated by changes in autonomic nervous system activity and/or hypothalamic–pituitary–adrenal (HPA) axis activity as we previously showed [3, 4]. Regarding aetiology of diabetes, it must be pointed out that such changes have been described in prediabetic states [13, 14]. For example, Pima Indians have a low sympathetic nervous system (SNS) activity, which may contribute to their increased risk of obesity and type 2 diabetes [15]. Furthermore, it is now assumed that, like glucose, NEFA or their metabolites could modulate neuronal activity as a means of directly monitoring ongoing fuel availability by CNS nutrient-sensing neurons involved in the regulation of energy homeostasis [16, 17].
C-fos immunostaining was performed to identify areas involved in NEFA effects and to provide an index of neural activity in those areas. Infusion of lipids/heparin for 24 h was associated with increased LH and decreased medial hypothalamic (ARC, VMH, PVN, DMH) numbers of expressing FLI neurons, indicating modifications of nervous activity in these areas. The decrease in FLI neurons observed in the arcuate nucleus of IL rats, which is associated with an increased HGP, is consistent with the results of studies indicating that neuronal activation of this area is a contributing factor in the decrease in HGP [16, 18]. In addition, the decreased number of FLI neurons in VMH indicated a lower activity in this nucleus, which is likely to promote a decreased sympathetic tone, as both VMH and ARC are known to activate the sympathetic nervous system via polysynaptic efferent pathways [19]. This is consistent with a study reporting a decrease in spontaneous neuronal activity in VMH subsequent to an injection of NEFA in this area [20]. Regarding LH, which is part of the hypothalamic area controlling parasympathetic activity, it has been reported to promote insulin secretion in the presence of a simultaneous rise in glucose [21].
Altogether, data suggest that NEFA could act at the level of central nervous system areas controlling SNS and/or HPA axis and consequently controlling insulin secretion and action [22].
Enzymes involved in NEFA metabolism, such as fatty acid synthase (FAS), have been shown to be present and active in hypothalamic neurons [23]. Furthermore, carnitine palmitoyl transferase I (CPT-I), a key enzyme in beta oxidation, is also expressed in some brain areas, including the hypothalamus [24], and its inhibition decreases food intake and hepatic glucose output [8]. Thus, the effects of a central NEFA overload could presuppose their beta oxidation. As the beta oxidation depends upon the entry of NEFA into the mitochondria through the CPT1, we infused etomoxir, an inhibitor of CPT1 into the right lateral ventricle. Neuronal activities in IL rats infused with etomoxir were normalised in ARC, LH, PVN and VMH, as assessed by c-fos immunostaining, and the effect of NEFA on GIIS and hepatic insulin sensitivity was blunted.
This is consistent with the results of some studies indicating that NEFA may alter the synthesis or the release of neuropeptides [25]. The effect of a NEFA overload could be partially mediated by an increase in ATP production, which could inactivate ATP-sensitive K+ channels and trigger cell exocytosis. In contrast, in the presence of a block of CPT1 activity during the lipid infusion, one could expect decreased ATP production and a concomitant increase in intracellular FA-CoA esters [8]. Such an increase could directly activate ATP-sensitive K+ channels as has been shown for oleyl-CoA [5, 26], opposing the inhibitory effects of ATP [27].
Etomoxir also decreased food intake by the same amount in IL rats and C rats. This is consistent with the results of studies revealing the potent anorectic effect of FAS inhibitors, a situation that increases malonyl coenzyme A (malonyl-CoA), an endogenous inhibitor of CPT1 [28, 29, 30]. This anorectic effect of etomoxir could be explained by the likely intracellular increase of NEFA subsequent to CPT1 inhibition, which acted as a central signal of “nutrient abundance” and activated a chain of neuronal events leading to food intake inhibition [8]. Elevated intracellular NEFA could also be responsible for the decrease of HGP [5], as a physiological way of reducing peripheral glucose availability. It is possible that the increased number of FLI neurons in VMH of C and IL rats treated with etomoxir is related to the decrease in food intake, as VMH activation is known to stimulate food consumption [31]. It should be pointed out that IL/heparin infusion alone does not lead to a decrease in food intake, consistent with the idea that, in this situation, NEFA oxidation prevails over the intracellular accumulation of FA-CoA. Finally, a decrease in food intake is not associated with a concomitant decrease in body weight; this could be related to changes in metabolic rate.
In conclusion, increased central NEFA oxidation is likely to be involved in the setting of insulin hypersecretion in response to glucose and hepatic insulin resistance, as the inhibition of beta oxidation blunted insulin hyper-response and restored normal hepatic glucose output. It is likely that when CPT1 is inhibited, NEFA accumulate in the cell and act as a satiety signal that inhibits food intake and decreases HGP. Thus, depending on their fate in the cell, i.e. oxidation or accumulation, NEFA could have adverse effects on energy homeostasis.
Abbreviations
- ARC:
-
arcuate nucleus
- CPT1:
-
carnitine palmitoyl transferase 1
- DMH:
-
dorsomedial hypothalamus
- FLI:
-
fos-like immunoreactivity
- GIIS:
-
glucose-induced insulin secretion
- ICV:
-
intracerebroventricular
- LH:
-
lateral hypothalamus
- PVN:
-
paraventricular nucleus
- VMH:
-
ventromedial hypothalamus
References
Unger RH, Orci L (2001) Diseases of liporegulation: new perspective on obesity and related disorders. Faseb J 15:312–321
Unger R (1995) Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 44:863–870
Magnan C, Collins S, Berthault M et al. (1999) Lipid infusion lowers sympathetic nervous activity and leads to increased ß-cell responsiveness to glucose. J Clin Invest 103:413–419
Clement L, Cruciani-Guglielmacci C, Magnan C et al. (2002) Intracerebroventricular infusion of a triglyceride emulsion leads to both altered insulin secretion and hepatic glucose production in rats. Pflugers Arch 445:375–380
Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L (2002) Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51:271–275
Obici S, Rossetti L (2003) Minireview: nutrient sensing and the regulation of insulin action and energy balance. Endocrinology 144:5172–5178
Havel PJ (2001) Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med (Maywood) 226:963–977
Obici S, Feng Z, Arduini A, Conti R, Rossetti L (2003) Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 9:756–761
Gilbert M, Magnan C, Turban S, Andre J, Guerre-Millo M (2003) Leptin receptor-deficient obese Zucker rats reduce their food intake in response to a systemic supply of calories from glucose. Diabetes 52:277–282
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, London
Das UN (2002) Is type 2 diabetes mellitus a disorder of the brain? Nutrition 18:667–672
Elmquist JK, Marcus JN (2003) Rethinking the central causes of diabetes. Nat Med 9:645–647
Chan O, Inouye K, Riddell MC, Vranic M, Matthews SG (2003) Diabetes and the hypothalamo-pituitary-adrenal (HPA) axis. Minerva Endocrinol 28:87–102
Jeanrenaud B, Rohner-Jeanrenaud F (2000) CNS-periphery relationships and body weight homeostasis: influence of the glucocorticoid status. Int J Obes Relat Metab Disord 24 [Suppl 2]:S74–S76
Tataranni PA, Young JB, Bogardus C, Ravussin E (1997) A low sympathoadrenal activity is associated with body weight gain and development of central adiposity in Pima Indian men. Obes Res 5:341–347
Obici S, Rossetti L (2003) Nutrient sensing and the regulation of insulin action and energy balance. Endocrinology 144:5172–5178
Seeley RJ, Woods SC (2003) Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci 4:901–909
Obici S, Zhang BB, Karkanias G, Rossetti L (2002) Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8:1376–1382
Ahren B, Taborsky G, Porte D (1986) Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia 29:827–836
Oomura Y (1976) Significance of glucose, insulin, and free fatty acid on the hypothalamic feeding and satiety neurons. In: D. Novin, W. Wyrwicka, G. Bray (eds) Hunger: basic mechanisms and clinical implications. Raven Press, New York, pp 145–157
Berthoud HR, Bereiter DA, Jeanrenaud B (1980) Role of the autonomic nervous system in the mediation of LHA electrical stimulation-induced effects on insulinemia and glycemia. J Auton Nerv Syst 2:183–198
Ahrén B (2000) Autonomic regulation of islet hormone secretion-implication for health and disease. Diabetologia 43:393–410
Kim EK, Miller I, Landree LE et al. (2002) Expression of FAS within hypothalamic neurons: a model for decreased food intake after C75 treatment. Am J Physiol Endocrinol Metab 283:E867–879
Sorensen A, Travers MT, Vernon RG, Price NT, Barber MC (2002) Localization of messenger RNAs encoding enzymes associated with malonyl-CoA metabolism in mouse brain. Brain Res Gene Expr Patterns 1:167–173
Yehuda S, Rabinovittz S, Carasso R, Mostofsky D (1998) Fatty acids and brain peptides. Peptides 19:407–419
Branstrom R, Aspinwall CA, Valimaki S et al. (2004) Long-chain CoA esters activate human pancreatic beta-cell KATP channels: potential role in Type 2 diabetes. Diabetologia 47:277–283
Gribble FM, Proks P, Corkey BE, Ashcroft FM (1998) Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA. J Biol Chem 273:26383–26387
Loftus TM, Jaworsky DE, Frehywot GL et al. (2000) Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288:2379–2381
Clegg DJ, Wortman MD, Benoit SC, McOsker CC, Seeley RJ (2002) Comparison of central and peripheral administration of C75 on food intake, body weight, and conditioned taste aversion. Diabetes 51:3196–3201
Wortman MD, Clegg DJ, D’Alessio D, Woods SC, Seeley RJ (2003) C75 inhibits food intake by increasing CNS glucose metabolism. Nat Med 9:483–485
Valensi P, Doare L, Perret G, Germack R, Paries J, Mesangeau D (2003) Cardiovascular vagosympathetic activity in rats with ventromedial hypothalamic obesity. Obes Res 11:54–64
Acknowledgements
We thank B. Hegarty and N. Oakes for providing the etomoxir. We also thank M. Gilbert for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cruciani-Guglielmacci, C., Hervalet, A., Douared, L. et al. Beta oxidation in the brain is required for the effects of non-esterified fatty acids on glucose-induced insulin secretion in rats. Diabetologia 47, 2032–2038 (2004). https://doi.org/10.1007/s00125-004-1569-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1569-2