Abstract
Aims
We investigated whether low subcutaneous thigh fat is an independent risk factor for unfavourable glucose and lipid levels, and whether these associations differ between sexes, and between white and black adults. Our secondary aim was to investigate which body composition characteristics (lean tissue, fat tissue) are reflected by anthropometric measures (waist and thigh circumference).
Methods
Anthropometric measurements and computed tomography of the abdomen and of the thigh were performed for all participants of the Health, Aging and Body Composition Study, who were aged 70–79 years. Fasting glucose, triglycerides and HDL-cholesterol, and 2-h postload glucose were determined.
Results
After excluding those already diagnosed with diabetes or dyslipidaemia, we analysed data from 2,106 participants. After adjustment for abdominal subcutaneous and visceral fat, and intermuscular thigh fat, larger thigh subcutaneous fat area was statistically significantly associated with lower ln-transformed triglycerides [standardised beta (95% CI) −0.12 (−0.20 to −0.04) in men and −0.13 (−0.21 to −0.05) in women] and higher ln-HDL-cholesterol [0.10 (0.02 to 0.19) and 0.09 (0.01 to 0.18), respectively]. The associations with lower glucose levels were strong in men [−0.11 (−0.20 to −0.02) for fasting and −0.14 (−0.23 to −0.05) for postload glucose], but not statistically significant in women [−0.02 (−0.10 to 0.07) and −0.04 (−0.13 to 0.05), respectively]. There were no differences in the associations between white and black persons. Waist circumference was more strongly associated with abdominal subcutaneous fat, and this association became stronger with increasing BMI, whereas the association with visceral fat became weaker. Thigh circumference was equally dependent on thigh fat and thigh muscle in men, whereas in women the fat component was the main contributor.
Conclusion
Larger subcutaneous thigh fat is independently associated with more favourable glucose (in men) and lipid levels (in both sexes) after accounting for abdominal fat depots, which are associated with unfavourable glucose and lipid levels. Anthropometric measures reflect different fat depots at different levels of BMI at the abdomen, and reflect both fat and lean tissue at the thigh. These results emphasise the importance of accurate measures of regional body composition when investigating potential health risks.
Similar content being viewed by others
Introduction
Waist and hip circumference have been shown to have independent and opposite associations with cardiovascular risk factors in Caucasian men and women. Larger waist circumference was associated with unfavourable glucose and lipid levels, whereas larger hip or thigh circumference was associated with more favourable levels [1–6]. Although visceral fat is thought to be involved [7], the exact underlying pathophysiological mechanisms remain unclear. This may be partly because not many studies include accurate assessments of body composition. By use of dual-energy X-ray absorptiometry (DXA) it has been shown that a larger waist circumference mainly represents more trunk fat rather than trunk lean mass, but a larger hip circumference represents both more leg fat mass and leg lean mass [8]. In addition, larger leg fat mass and larger leg lean mass was associated with lower insulin and glucose levels, after adjustment for trunk fat and lean mass [8, 9].
Because abdominal and femoral fat depots have different lipolytic activity [10, 11], femoral fat tissue may be more likely to take up non-esterified fatty acids (NEFA) from the circulation. As a result, the gluteal–femoral fat depots protect the liver, pancreas, and skeletal muscle from high NEFA exposure and accumulation, which is related to the development of insulin resistance and beta-cell dysfunction [12–18]. Unfortunately, DXA does not allow separate quantification of intermuscular and subcutaneous fat in the legs, and of visceral and subcutaneous fat in the trunk.
In the Health, Aging and Body Composition Study investigators have found that more intermuscular fat in the thigh determined by computed tomography (CT) was significantly associated with a worse glucose tolerance status, whereas subcutaneous fat in the thigh was not [19]. In these analyses, however, abdominal fat was not taken into account and the independent role of thigh fat depots was not studied. Potential differences between races in the relationship of the different fat depots to glucose metabolism were not reported, which may be relevant considering the differences in body composition and cardiovascular disease risk between black and white people.
The aim of the current study was to investigate independent associations of CT measured body composition characteristics with glucose and lipid levels, in subjects without known diabetes or dyslipidaemia. In addition, we investigated whether these associations differed between men and women, and between black and white people. We hypothesised that low subcutaneous thigh fat would be independently associated with unfavourable glucose and lipid levels. Our secondary aim was to investigate which body composition characteristics (lean tissue, different fat depots) are reflected by anthropometric measures (waist and thigh circumference) in the elderly.
Materials and methods
Subjects
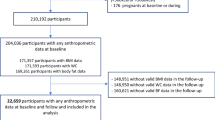
The study population consisted of 3,075 well-functioning black and white men and women aged 70–79 years participating in the Health, Aging, and Body Composition (Health ABC) Study. White participants were recruited from a random sample of Medicare beneficiaries residing in zip codes from the metropolitan areas surrounding Pittsburgh, PA, USA and Memphis, TN, USA. Black participants were recruited from all age-eligible residents in these geographic areas. Eligibility criteria included: age 70–79 years in the recruitment period from March 1997 to July 1998, self-report of no difficulty walking one quarter of a mile or climbing ten steps without resting, no difficulty performing basic activities of daily living, no reported use of a cane, walker, crutches or other special equipment to get around, no history of active treatment for cancer in the prior 3 years, and no plan to move out of the area in the next 3 years. The population comprised 41.7% African–American, 58.3% Caucasian, and 48.5% male. The experimental procedures were approved by the Human Investigation and Review Boards at the University of Pittsburgh at Pittsburgh and the University of Tennessee at Memphis. Written informed consent was obtained from all subjects.
In the present study, subjects for whom fasting glucose (n=29) or abdominal or thigh CT (n=154) data were missing were excluded from all analyses. Also, persons who reported previous diabetes diagnosis (n=468, of which 385 persons were using glucose-lowering medication) or who were using lipid-lowering medication (n=437) were excluded, because treatment (either diet or medication) could possibly influence the associations under consideration. Finally, 2,106 subjects were studied in the analyses.
Body composition
Body weight was measured to the nearest 0.1 kg with a standard balance beam scale. Height was measured barefoot to the nearest 0.1 cm using a Harpenden stadiometer (Holtain, UK). BMI was calculated as weight divided by height squared (kg m−2). Abdominal circumference (cm) was measured with a flexible plastic tape measure to the nearest 0.1 cm at the level of the largest circumference (seen from the side), at the end of expiration, between the lower rib and the iliac crest, while subjects were standing with their weight equally distributed on both feet, arms at their sides, and head facing straight forward. Thigh circumference (cm) was measured at the mid-thigh between the inguinal crease and the proximal border of the patella. A total body DXA scan was performed to measure total body fat using fan-beam technology (Hologic QDR4500A, software version 8.21; Hologic, Waltham, NY, USA). CT scans of the abdomen and thighs were acquired in Memphis using a Somatom Plus 4 (Siemens, Erlangen, Germany) or a Picker PQ 2000S (Marconi Medical Systems, Cleveland, OH, USA), and in Pittsburgh using a 9800 Advantage (General Electric, Milwaukee, WI, USA) as described previously [20, 21]. Briefly, the scans were completed at 120 kVp, 200–250 mA s, and slice thickness was set at 10 mm. For the scan of the abdomen at L4/L5 level, subjects were placed in the supine position with their arms above their head and legs elevated with a cushion to reduce the curve in the back. The scan at mid-thigh level was performed at one half of the distance between the medial edge of the greater trochanter and the intercondyloid fossa. The images were transferred to a Sun workstation (SPARCstation II; Sun Microsystems, Mountainview, CA, USA) for determination of adipose and muscle tissue areas using IDL-based (RSI Systems, Boulder, CO, USA) software developed at the reading centre. Fat tissue, muscle tissue and bone were distinguished by their particular range of tissue density in Hounsfield units (HU). Soft tissue type was determined using the bimodal image distribution histogram resulting from the distribution of HU values in fat tissue and muscle tissue [22]. Visceral fat tissue was manually distinguished from subcutaneous fat tissue by tracing along the fascial plane defining the internal abdominal wall. For the muscle area of the abdomen, areas of the left and right psoas, rectus, and lateral abdominal muscles were added. In the thighs, intermuscular and visible intramuscular fat tissue were separated from subcutaneous adipose tissue by drawing a line along the deep fascial plane surrounding the thigh muscles. The total area of non-adipose, non-bone tissue within the deep fascial plane was used as a measure of muscle area. Areas of the left and right thigh were added.
Metabolic variables and lifestyle
Serum triglycerides and HDL-cholesterol were determined on a Vitros 950 analyser (Johnson & Johnson, Rochester, NY, USA) after an overnight fast. A 75-g oral glucose tolerance test was performed in subjects without previously diagnosed diabetes. Participants were considered to have previously diagnosed diabetes if they reported having diabetes or were using glucose-lowering medication. Fasting and 2-h postload plasma glucose were measured by use of an automated glucose oxidase reaction (YSI 2300 Glucose Analyser, Yellow Springs, OH, USA).
Smoking status (never, current, former), pack-years exposure to cigarettes, drinking history (never, current, former) and current drinking status (no, less than once a week, one to seven times a week, more than one a day) and physical activity (in the past 7 days) were assessed by means of an interviewer-administered questionnaire. The time spent on gardening, heavy chores, light house work, grocery shopping, laundry, climbing stairs, walking for exercise, walking for other purposes, aerobics, weight or circuit training, high-intensity exercise activities and moderate-intensity exercise activities was obtained, as also was information on the intensity level at which each activity was performed. A metabolic equivalent value was assigned to each activity/intensity combination and was used to calculate the number of kilocalories per week per kilogram of body weight spent on that activity [23]. For each participant the scores of all performed activities were summed and multiplied by body weight to create an overall physical activity score in kilocalories per week. Similarly, a summary score of performed exercise activities was created, which included aerobics, weight training, medium and high-intensity exercise activities and walking for exercise.
Statistical methods
All analyses were performed by use of SPSS for Windows version 10.1 (SPSS, Chicago, IL, USA). To study the association between each body composition measure by CT (independent variable) and glucose and lipid levels (dependent variables), linear regression analyses were performed, adjusting for age, site (Pittsburgh or Memphis) and race. Subsequently, the model was also adjusted for the other body composition variables (all forced into the model). Effect modification by sex and race was evaluated by adding product terms to the regression models. Effect modification by BMI was evaluated by stratification, because in this case adding product terms disturbed the models because of multi-colinearity. Confounding by lifestyle factors and muscle area was evaluated by adding these variables to the regression models.
In addition, similar regression analyses were performed to study independent associations between body composition measures by CT (independent variables) and anthropometrically derived waist and hip circumferences (dependent variables).
To facilitate direct comparisons, results of the regression analyses are reported as standardized betas (standardised regression coefficients). A standardized beta of 0.1 indicates that when the independent variable increases by 1 SD, the dependent variable increases by 0.1 SD. We considered the stability of the regression models to be disturbed by multi-colinearity if the tolerance was <0.1. The tolerance is a statistic used to determine how much the independent variables are linearly related to one another. It is calculated as 1 minus R-squared for an independent variable when it is predicted by the other independent variables already included in the model.
Results
Characteristics are shown in Table 1, by gender and race. Despite higher or similar BMI and subcutaneous fat in black people compared with white people, black adults have lower visceral fat compared with white adults. Table 2 shows associations of each body composition variable with fasting and 2-h glucose levels, and with ln-transformed triglycerides and HDL-cholesterol, whereas Table 3 shows the associations additionally adjusted for the other body composition variables. Larger total abdominal fat area was independent of thigh fat area associated with unfavourable levels of all these glucose and lipid levels, whereas larger total thigh fat area was associated with more favourable levels after adjustment for total abdominal fat area (Model 1), in both men and women. Subdivision of the fat areas revealed that the associations with total thigh fat area were mainly determined by the subcutaneous thigh fat area (Model 2) whereas the associations of total abdominal fat were mainly determined by visceral fat area (Model 3). However, if visceral fat is taken into account, abdominal subcutaneous fat is also associated with unfavourable glucose and lipid levels. If all four fat depots were included in one model (Model 4), larger thigh subcutaneous fat area was independently associated with more favourable glucose and lipid levels, except for glucose levels in women. We also added abdominal and thigh subcutaneous fat areas to calculate total subcutaneous fat. If total subcutaneous fat was added to the regression model which already included visceral fat and intermuscular thigh fat, it was not significantly related to glucose and lipid levels (data not shown), confirming opposite associations of different regional subcutaneous fat depots with these variables. There was no statistically significant effect modification by race, except for the association of thigh subcutaneous fat area with HDL-cholesterol in men in Model 2 and Model 4, which was present in white men, but not in black men (standardised beta of 0.20 in white men and −0.04 in black men in Model 4). In all models of Table 3, additional adjustment for abdominal or thigh muscle area did not materially change the results, and a larger muscle area was not associated with glucose and lipid levels in most cases, or was, in a few cases, statistically significantly associated with less favourable glucose and lipid levels. Also, adjustment for height, lifestyle variables (smoking, alcohol intake, and physical activity), or oral estrogen use did not change the results. When we only selected subjects younger than 75 years old, the associations remained similar. Also excluding subjects using oral oestrogens (two men and 249 women) did not change the results. Stratification by BMI group did not show clear trends of increasing or decreasing associations with higher BMI group. None of the regression models showed evidence of multi-colinearity by statistical testing.
To visualize the meaning of the opposite associations that we found in Table 3, as an example, mean fasting glucose levels in men are shown in Fig. 1, after stratification of the male population in (race-specific) tertiles of visceral and subcutaneous thigh fat, adjusted for site, age, race, abdominal subcutaneous fat and intermuscular thigh fat. It is clearly shown that with a larger visceral fat area, glucose levels are higher. In contrast, within each tertile of visceral fat, with a larger subcutaneous thigh fat area, glucose levels are lower.
In both sexes waist circumference was more strongly associated with abdominal fat area than with abdominal muscle area (Table 4). When visceral fat and subcutaneous fat from the abdomen were examined separately, the subcutaneous compartment seemed the strongest determinant of waist circumference. Stratification by (gender- and race-specific) BMI tertiles revealed that the association of waist circumference with abdominal subcutaneous fat became stronger, and with visceral fat became weaker, with higher BMI group, particularly in men (Fig. 2). The thigh circumference was equally dependent on thigh fat and thigh muscle area in men, whereas in women the fat component was the main contributor. Intermuscular fat area made a relatively small contribution. In particular the association of the subcutaneous thigh fat area, not the intermuscular fat area, with thigh circumference strengthened with higher BMI group. There was no statistically significant (p>0.05) effect modification by race, except for the associations of waist circumference with abdominal subcutaneous fat and with muscle area, which were stronger for black men than for white men (standardised betas were, respectively, 0.61 and 0.41 for subcutaneous fat and 0.15 and 0.07 for muscle).
Independent associations (regression coefficients) of CT visceral fat area and CT abdominal subcutaneous fat area (adjusted for each other) with anthropometrically derived waist circumference, adjusted for CT abdominal muscle area, age, site (Pittsburgh or Memphis), and race, stratified by tertiles of BMI and separately for men (a) and women (b)
Discussion
Subcutaneous thigh fat was, independently of abdominal fat depots, related to more favourable levels of glucose and lipids in black and white subjects, except to glucose levels in women. This paper also showed that waist circumference was related to both visceral fat and subcutaneous fat, and that waist circumference better represented visceral fat at a low BMI. Apart from reflecting subcutaneous thigh fat, thigh circumference also represented muscle, in particular in men.
This study has some limitations. First, because we investigated a relatively healthy and well-functioning population, we may have underestimated the true associations. The results should therefore not be extended to the general population. Second, because of the cross-sectional design of the study, causality cannot be assumed. Longitudinal analyses, however, are also limited because body composition values are not stable over time, particularly in the elderly. Finally, the anthropometric analyses were performed in people without known diabetes or dyslipidaemia, which could also create a bias when generalizing to the total population.
Visceral fat was the strongest independent correlate of unfavourable glucose and lipid levels, which supports the hypothesis that visceral fat, in particular, contributes to the higher non-esterified fatty acid (NEFA) levels, which are directly released in the portal vein leading to the liver. In the liver, NEFA play an important role in the development of insulin resistance, by reducing hepatic insulin clearance, increasing gluconeogenesis and increasing dyslipidaemia [7, 24]. In addition, because of increased NEFA levels, NEFA also accumulate in non-adipose tissue (i.e. ectopic fat storage) such as muscle, the pancreas and the liver, which contributes to and exacerbates insulin resistance and type-2 diabetes [13–18]. We found that abdominal subcutaneous fat was also related to unfavourable glucose and lipid levels, even after adjustment for visceral fat area. This may indicate that also abdominal subcutaneous fat contributes to higher NEFA levels.
In contrast with abdominal subcutaneous fat, subcutaneous thigh fat was independently related to more favourable levels of glucose and lipid levels, which confirmed our hypothesis of low subcutaneous fat as a risk factor. It has been proposed that subcutaneous thigh fat acts as a “metabolic sink” for circulating NEFA [12]. Because of differences in lipolytic activity between abdominal subcutaneous fat and subcutaneous thigh fat [10, 11], subcutaneous thigh fat is more likely to take up NEFA from the circulation, and therefore protects other organs against high NEFA exposure. In this manner, ectopic fat storage is prevented which leads to a lower risk of insulin resistance [12, 13].
Fat cells are known to secrete many signalling factors, some of which may be involved in the development of insulin resistance [25]. Examples include leptin, adiponectin, interleukin-6, tumour necrosis factor-alpha, plasminogen activator inhibitor-1, and many more. There are known regional differences in the secretion of leptin, adiponectin and interleukin-6 between visceral and abdominal subcutaneous fat [26–28]. It might be possible that there are also regional differences in secretion of these adipokines between abdominal subcutaneous and subcutaneous thigh fat, which could contribute to the different associations of these fat depots with glucose and lipid levels. More research in this area is clearly needed.
The independent relationship between larger leg fat mass measured by DXA and more favourable glucose and lipid level variables has been found in a number of studies, in both middle-aged and elderly subjects [1, 8, 9]. When we used the DXA measurements from the Health ABC Study, we observed similar associations (unpublished results). The limitation of DXA is that it cannot distinguish between visceral and abdominal subcutaneous fat in the trunk, and between intermuscular and subcutaneous fat in the legs. By use of CT, we found that the protective association of DXA leg fat with glucose and lipid levels was because of the subcutaneous thigh fat, and not intermuscular thigh fat. Previously, in a small group of obese men (aged 29–42 years), femoral adipose tissue by CT was also found to be negatively associated with triglycerides and positively with HDL-cholesterol [29], and similar results were found in women [30]. Also, in a small sample of black women leg fat seemed to be independently related to better lipid levels [31]. In the current work we confirmed these observations using CT measurements in a large elderly population, including both black and white people, and extended the observations by including glucose levels as outcome measures.
Previously, we found larger leg lean mass by DXA (which is mainly muscle mass) to be related to lower glucose levels, independent of trunk fat and leg fat mass [8], which could also be confirmed in the Health ABC Study when we used DXA measurements (unpublished results). However, we did not observe any relationship with thigh muscle area if using CT data, after adjustment for abdominal and thigh fat depots. CT and DXA generally agree in measuring muscle and fat mass, if similar regions are compared [32, 33]. However, it is possible that the thigh muscle area from a single-slice CT scan is not representative of the muscle mass of the total leg as is measured by DXA. Similarly, this could also be the reason for not finding a relationship with intermuscular fat. More detailed measurements of total muscle might be needed to appropriately assess the relationship with the metabolic profile.
There was a difference between black and white participants in body composition in our study, which is also known from previous studies [34–36]. Black persons have generally less visceral fat compared with white persons, whereas the subcutaneous fat (either abdominal or at the thigh) is higher, for any level of total body fat. Black people had a better lipid profile in our data, which has also been found previously [36, 37]. It has been shown that in both sexes black persons have higher LPL activity and lower HL activity compared with white persons [36], which may explain these differences in lipid profile and fat distribution. It has been suggested that because of these differences between enzyme activity, black persons are more likely to store their lipids in subcutaneous fat depots, which in turn will lead to a better lipid profile. However, we did not find a statistically significant difference between races in the relationship between different fat depots and glucose and lipid levels. The difference between black and white people in glucose and lipid levels might be explained by the relative amounts of different fat depots, rather than by the relationship between these fat depots and glucose and lipid levels per se.
WHR is generally used as measure of abdominal fat distribution, presumably reflecting visceral fat [38, 39]. A higher WHR, however, can also be caused by a smaller hip circumference. By use of DXA measurements it has recently been shown that the hip circumference not only represents fat accumulation in the legs, but is also related to the lean mass in the legs [8]. In the same study the waist circumference mainly reflected fat mass in the trunk. It remained unclear whether this was mainly caused by the visceral or subcutaneous fat depot. In the present paper we show that abdominal subcutaneous fat depot mainly determines the waist circumference, particularly in persons with a higher BMI. Clearly, the WHR does not simply represent visceral fat accumulation only. It has been suggested that increased abdominal fat accumulation may be less hazardous in older than in younger persons, because anthropometric measures of abdominal obesity were not related to (cardiovascular) mortality in the elderly, and it has been shown that lipolysis in visceral fat, which causes free fatty acid flux, is reduced with ageing [40]. Our study shows, however, that if more precise measures of body composition are taken, visceral fat is still hazardous in older adults.
In conclusion, high visceral fat and high abdominal subcutaneous fat are both independently associated with unfavourable glucose and lipid levels. In contrast, high subcutaneous thigh fat is independently associated with more favourable glucose (in men) and particularly lipid levels (in both sexes), if the abdominal fat depots are taken into account. These results underline the importance of accurate measures of regional body composition in health-risk research. Further research is needed to elucidate underlying pathophysiological mechanisms. Waist circumference reflects different fat depots at different levels of BMI, and thigh circumference reflects both fat and muscle tissue. Caution is needed when interpreting anthropometric measurements.
Abbreviations
- CT:
-
computed tomography
- DXA:
-
dual-energy X-ray absorptiometry
- HU:
-
hounsfield units
References
Terry RB, Stefanick ML, Haskell WL, Wood PD (1991) Contributions of regional adipose tissue depots to plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism 40:733–740
Seidell JC, Perusse L, Despres JP, Bouchard C (2001) Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec family study. Am J Clin Nutr 74:315–321
Snijder MB, Dekker JM, Visser M et al (2003) Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes Res 11:104–111
Snijder MB, Dekker JM, Visser M et al (2003) Associations of hip and thigh circumferences independent of waist circumference with the incidence of type-2 diabetes: the Hoorn Study. Am J Clin Nutr 77:1192–1197
Snijder MB, Zimmet PZ, Visser M, Dekker JM, Seidell JC, Shaw JE (2004) Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab study. Int J Obes Relat Metab Disord 28:402–409
Lissner L, Bjorkelund C, Heitmann BL, Seidell JC, Bengtsson C (2001) Larger hip circumference independently predicts health and longevity in a Swedish female cohort. Obes Res 9:644–646
Bjorntorp P (1990) “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 10:493–496
Snijder MB, Dekker JM, Visser M et al (2004) Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care 27:372–377
Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM (2002) Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab 282:E1023–E1028
Rebuffe-Scrive M, Enk L, Crona N et al (1985) Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J Clin Invest 75:1973–1976
Rebuffe-Scrive M, Lonnroth P, Marin P, Wesslau C, Bjorntorp P, Smith U (1987) Regional adipose tissue metabolism in men and postmenopausal women. Int J Obes 11:347–355
Frayn KN (2002) Adipose tissue as a buffer for daily lipid flux. Diabetologia 45:1201–1210
Ravussin E, Smith SR (2002) Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type-2 diabetes mellitus. Ann N Y Acad Sci 967:363–378
Tiikkainen M, Tamminen M, Hakkinen AM et al (2002) Liver-fat accumulation and insulin resistance in obese women with previous gestational diabetes. Obes Res 10:859–867
Seppala-Lindroos A, Vehkavaara S, Hakkinen AM et al (2002) Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87:3023–3028
Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC (2003) Fatty liver in type-2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 285:E906–E916
Kelley DE, Goodpaster BH (2001) Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24:933–941
McGarry JD (2002) Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type-2 diabetes. Diabetes 51:7–18
Goodpaster BH, Krishnaswami S, Resnick H et al (2003) Association between regional adipose tissue distribution and both type-2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 26:372–379
Snijder MB, Visser M, Dekker JM et al (2002) The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: a comparison with computed tomography and anthropometry. Int J Obes Relat Metab Disord 26:984–993
Visser M, Kritchevsky SB, Goodpaster BH et al (2002) Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging and Body Composition Study. J Am Geriatr Soc 50:897–904
Seidell JC, Oosterlee A, Thijssen MA et al (1987) Assessment of intra-abdominal and subcutaneous abdominal fat: relation between anthropometry and computed tomography. Am J Clin Nutr 45:7–13
Ainsworth BE, Jacobs DR Jr, Leon AS (1993) Validity and reliability of self-reported physical activity status: the Lipid Research Clinics questionnaire. Med Sci Sports Exerc 25:92–98
Despres JP, Lemieux S, Lamarche B et al (1995) The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int J Obes Relat Metab Disord 19(Suppl 1):S76–S86
Jazet IM, Pijl H, Meinders AE (2003) Adipose tissue as an endocrine organ: impact on insulin resistance. Neth J Med 61:194–212
van Harmelen V, Dicker A, Ryden M et al (2002) Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes 51:2029–2036
Eriksson P, Van Harmelen V, Hoffstedt J et al (2000) Regional variation in plasminogen activator inhibitor-1 expression in adipose tissue from obese individuals. Thromb Haemost 83:545–548
Motoshima H, Wu X, Sinha MK et al (2002) Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab 87:5662–5667
Pouliot MC, Despres JP, Nadeau A et al (1992) Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 41:826–834
Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS (1997) Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr 65:855–860
Hunter GR, Giger JN, Weaver M, Strickland OL, Zuckerman P, Taylor H (2000) Fat distribution and cardiovascular disease risk in African–American women. J Natl Black Nurses Assoc 11:7–11
Visser M, Fuerst T, Lang T, Salamone L, Harris TB (1999) Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study—Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol 87:1513–1520
Salamone LM, Fuerst T, Visser M et al (2000) Measurement of fat mass using DEXA: a validation study in elderly adults. J Appl Physiol 89:345–352
Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R (1996) Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism 45:1119–1124
Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER (1999) Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (coronary artery risk development in young adults) study. Am J Clin Nutr 69:381–387
Despres JP, Couillard C, Gagnon J et al (2000) Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the health, risk factors, exercise training, and genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol 20:1932–1938
Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX (1997) Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes 46:456–462
Seidell JC, Bouchard C (1997) Visceral fat in relation to health: is it a major culprit or simply an innocent bystander? Int J Obes 21:626–631
Molarius A, Seidell JC (1998) Selection of anthropometric indicators for classification of abdominal fatness—a critical review. Int J Obes Relat Metab Disord 22:719–727
Seidell JC, Visscher TL (2000) Body weight and weight change and their health implications for the elderly. Eur J Clin Nutr 54(Suppl 3):S33–S39
Acknowledgements
This study was supported by NIA NO1-AG-6-2101, NO1-AG-6-2103 and NO1-AG-6-2106. All authors declare that they had no duality or conflicts of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Snijder, M.B., Visser, M., Dekker, J.M. et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 48, 301–308 (2005). https://doi.org/10.1007/s00125-004-1637-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1637-7