Abstract
Aims/hypothesis
The aim of this study was to establish the incidence of type 1 and type 2 diabetes mellitus in children aged 0–14 years.
Methods
The New Zealand Paediatric Surveillance Unit sought monthly reporting of diabetes mellitus cases from paediatricians. All resident children aged below 15 years (1996 census risk population 832,000) who met the criteria for diagnosis of diabetes mellitus from 1 January 1999 to 31 December 2000 were included. The average annual incidence of type 1 and type 2 diabetes was calculated, as were incidence rates according to age, sex, region, ethnicity and season. Case ascertainment was estimated using hospital admission data.
Results
There were 315 valid reports of new cases of diabetes. Of these, 298 (94.6%) had type 1 diabetes, 12 (3.8%) had type 2 diabetes and five had other specified types of diabetes. The average annual incidence of type 1 diabetes was 17.9/100,000 (95% CI: 15.9–20/100,000). Children in the South Island had a 1.5-fold higher incidence than children in the North Island, which was largely accounted for by the variation in incidence with ethnicity, in that the European rate was 4.5 times higher than the Maori rate. The average annual incidence of type 2 diabetes was 0.84/100,000 (95% CI: 0.37–1.26/100,000). Estimated case ascertainment rate was 95.2%.
Conclusions/interpretation
Type 1 diabetes incidence has doubled over the past three decades. The geographical differences previously described have persisted, and are largely explained by the ethnic variation in incidence. This population includes young adolescents with type 2 diabetes. These findings are in keeping with international trends.
Similar content being viewed by others
Introduction
Reports from many populations indicate that the incidence of both type 1 and type 2 diabetes is increasing in the under-15 age group [1, 2]. While increasing rates of obesity and sedentary lifestyles appear to account for the increase in type 2 diabetes, relatively little is known about why type 1 diabetes is increasing. Since the earliest registers of type 1 diabetes, it has been evident that a large (at least 35-fold) geographical difference in incidence exists, which has persisted until today [3]. Although these differences are considered to result from varying distributions of genetic and environmental risk factors, the exact variations and contributions of these risk factors remain unknown.
There have been two previous national incidence studies of diabetes mellitus in New Zealand children [4, 5]. The first reviewed hospital admission data for the 5 years 1968–1972 [4]. The average annual incidence for those aged 0–15 years was 8.9/100,000. Those living in the South Island had a statistically significant 1.4 times higher risk of diabetes than those living in the North Island. Ten years later, voluntary reporting by paediatricians and physicians for the four years 1978–1982 was sought [5]. The average annual incidence was 6.26/100,000 for those under 16 years, but this study was flawed because of significant under-reporting. Since then, only regional data have been available from the Auckland and Canterbury registers [6–10]. In Auckland, the average annual incidence was 9.3/100,000 during the years 1977–1984 [6], which is comparable to the North Island rate (8.1/100,000 for children aged 0–15 years) reported for the years 1968–1972. In Canterbury, the average annual incidence was 12.8/100,000 during the years 1982–1990, with regression models indicating no significant increase in incidence over time [8], while an average annual incidence of 24/100,000 was reported during the years 1990–1992 [9].
Dramatic increases in type 1 diabetes and type 2 diabetes elsewhere, a long period since previous New Zealand data, and anecdotal reports of type 2 diabetes in New Zealand children motivated the present study. We sought to determine the incidence of type 1 and type 2 diabetes in children and adolescents below 15 years of age and the influence of age, sex, ethnicity, region, season and family history on incidence of the disease.
Subjects and methods
The New Zealand Paediatric Surveillance Unit (NZPSU) was established in 1997 for the monitoring of acute flaccid paralysis, and to provide a system that allowed national surveillance of uncommon childhood conditions. Diabetes mellitus was included in the NZPSU monthly reporting system for the years 1999 and 2000.
Case definition
For inclusion, a child had to be below (up to but not including) 15 years of age and resident in New Zealand, with at least one of the following: (1) a random blood glucose measurement of 11.1 mmol/l or higher and presence of classical symptoms; (2) a fasting blood glucose of 7.1 mmol/l or higher; or (3) a blood glucose of 11.1 mmol/l or higher at 2 h during an OGTT or 2 h after a high-carbohydrate breakfast. Cases secondary to drugs or stress, for example sepsis, were excluded.
A questionnaire was sent to each reporting paediatrician seeking case demographics and information that would aid the differentiation of type 1 and type 2 diabetes. Specifically, the latter included ethnicity, family history of type 1 and type 2 diabetes in first-degree relatives, diabetes-related antibodies, growth parameters at the first outpatient appointment following diagnosis, the presence of acanthosis nigricans, and treatment regime. The questionnaire allowed for more than one ethnicity to be recorded. For purposes of analysis, ethnicity data were prioritised according to census prioritisation rules (http//www.statisticsnz.govt.nz), assigning each case to one ethnic group in the instance of multiple responses. For cases where questionnaire data indicated features suggestive of possible type 2 diabetes, clarification of the type of diabetes was sought from the paediatrician providing ongoing care, by asking for the current working diagnosis. Clarification was sought at least 12 months following diagnosis, allowing time for evolution of the diabetes course and for additional investigations.
Case ascertainment
At the end of the study period, lead paediatricians at each regional centre were sent a list of the cases noted from their region during the study, and asked to check and note any missing cases.
When standards for diabetes registers were established in 1983, it was decided that at least 90% case ascertainment for the primary source of data should be achieved [11]. The secondary data source used in this study was all hospital discharges of patients below 15 years of age containing a diabetes code for the years 1999 and 2000, which was independent of the NZPSU reporting system and was purchased from the New Zealand Health Information Service (NZHIS), Ministry of Health. Within this dataset, cases reported through the NZPSU were firstly identified. Next, from the list of hospital patients that remained, those with a diagnosis date prior to 1 January 1999 were identified using the hospital electronic database system (Ora*Care). Finally, for the few remaining ‘possibly missed’ cases, diabetes nurse educators were contacted to verify both the validity of the diagnosis and date of diabetes onset in order to determine which cases were actually missed. Case ascertainment was calculated using the capture–recapture method [12].
Statistical methods
The at-risk population was estimated from the 1996 census data and includes those below 15 years of age and resident in New Zealand. To allow direct comparison with international registries, incidence rates were calculated as the incidence per calendar year per 100,000 individuals at risk, according to protocols of the DIAMOND study [1].
Geographical regions were defined according to the census regional council area boundaries.
Incidence rates were age standardised using 5-year intervals (0–4, 5–9 and 10–14 years) and the direct method with a standard population consisting of equal numbers of children in each of the three groups (33%, 33% and 34% respectively). This method is the world population standard and has been used by the DERI study group [11] and the WHO DIAMOND project [1].
The Poisson distribution was assumed for the calculation of 95% confidence intervals around incidence rates [11]. Poisson regression models were used to identify differences in incidence rates by age, sex, geographical location, ethnicity and season. In the model for seasonal effect, the terms cos (ωt) and cons (ωt) were used (where ω=2π/365). The models were fitted using the SAS Genmod procedure [13].
Statistical calculations of data other than incidence rates used descriptive statistical methods. Continuous variables approximating a normal distribution were analysed using the Student’s two-tailed t-test, while those not normally distributed were analysed using the Mann–Whitney test. Categorical variables were analysed using the chi square test (Fisher’s exact). All p values are two-tailed and considered statistically significant if less than 0.05. Where means are reported, results are expressed as means (SD).
Ethical approval
The NZPSU regularly obtains ethical approval for the use of its anonymous reporting system for all conditions under investigation. In addition, investigators of each study are required to gain approval from their local ethics committee. The Otago Ethics Committee granted ethical approval for this study.
Results
There were 372 notifications of diabetes mellitus to the NZPSU for the 24-month period January 1999 to December 2000. This comprised 315 valid cases, 47 duplicates and ten errors. Of the 315 valid cases, 152 were diagnosed in 1999 and 163 in 2000; 298 (94.6%) had type 1 diabetes, 12 (3.8%) had type 2 diabetes and five (1.6%) had other specific types of diabetes and there were no cases of gestational diabetes. The cases with other specific types of diabetes included two probable cases of MODY, one case of cystic-fibrosis-related diabetes, one case of diabetes associated with a mitochondrial disorder and one child with Prader–Willi syndrome.
Case ascertainment
Of the 315 cases reported through the NZPSU, 301 were identified in the hospital discharge data. In addition, another 15 were identified from the hospital discharge data and verified by diabetes educators as valid cases (14 with type 1 diabetes and one case of MODY). Using the capture–recapture method, the estimated total number of new cases for the 2-year period was 330.7 (95% CI: 301–360.4), equating to an estimated case ascertainment level of 95.2%. When considering type 1 diabetes cases only, the estimated total number of new cases was 313.4 (95% CI: 290.6–336.2), again with an estimated case ascertainment level of 95.2%.
Type 1 diabetes mellitus
Of the 298 cases of type 1 diabetes, 142 were diagnosed in 1999 and 156 in 2000. The average annual incidence was 17.9 per 100,000 (95% CI: 15.9–20.0) children and adolescents under 15 years of age. The annual incidence rates were 17.1 and 18.7 per 100,000 for 1999 and 2000, respectively. Incidence rates were essentially unchanged after direct age standardisation. Table 1 summarises the incidence rates for various age groups according to sex.
Sex
The male:female ratio was 0.91:1, with that of the general under 15 population being 1.05:1. No statistically significant sex differences were found.
Age
Twenty-one percent of the children were below 5 years old. The incidence rate ratio for 5- to 14-year-olds compared with 0- to 4-year-olds was 1.7 (95% CI: 1.28–2.24). The median age at diagnosis was 9.5 years for males and 9 years for females, with peak incidence seen at 9–11 years.
Geographical residence and ethnicity
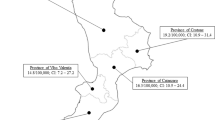
The geographical variation in incidence is depicted in Fig. 1. Gisborne and the West Coast are the least populated regions of the North and South Islands respectively (with 12,705 and 7,761 children of 0–14 years old, respectively) and there were no reported cases from either region. The incidence rate ratio for South Islanders compared with North Islanders was 1.55 (95% CI: 1.21–1.99).
Table 1 also presents the incidence rates according to ethnicity. Maori, Pacific Islanders and Asians all had significantly lower incidence rates (both absolute and relative to their respective population proportions) than Europeans. For example, while 23.6% of the population in this age group identify themselves as Maori, only 7.4% of the diabetes cases identified themselves as Maori.
Poisson regression models initially showed that the southern region (the majority of the South Island) had an incidence rate ratio of 1.43 (95% CI: 1.05–1.93) compared with the northern region (upper North Island). When the regression models included terms for both region and ethnicity, the incidence rate ratio for the southern region compared with the northern region was 1.04 (95% CI: 0.77–1.42).
Season
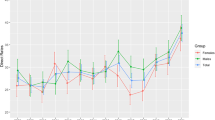
Figure 2 depicts the seasonal variation in incidence seen during the 2-year period and evident in each of the 2 years. Poisson regression models revealed that the seasonal variation in incidence was significant in each of the 2 years (p<0.0001).
Family history
Twenty-five cases (8.8%) had at least one first-degree relative also with type 1 diabetes, while another seven (2.5%) had a first-degree relative with type 2 diabetes. The mean ages at diagnosis for those with and without a first-degree relative with type 1 diabetes were 10.19 (3.5) and 8.29 (3.9) years, respectively (p=0.02).
Clinical presentation
Of the biochemical parameters taken on hospital presentation, the median (range) blood glucose and pH levels were 24.7 (4.5–73) mmol/l and 7.36 (6.93–7.55) mmol/l respectively. For those where a blood gas analysis was available (220), only 5% had evidence of severe diabetic ketoacidosis (pH below 7.1), while another 24% had evidence of mild to moderate diabetic ketoacidosis (pH 7.1–7.29). Assuming that all those with no blood gas data had no clinical evidence of diabetic ketoacidosis, less than 4% of type 1 diabetes cases presented with evidence of severe diabetic ketoacidosis.
Those with a family history of type 1 diabetes had significantly shorter duration of symptoms, lower blood glucose, higher pH, and higher bicarbonate levels (data not shown). No case with a first-degree relative with type 1 diabetes presented with severe diabetic ketoacidosis.
Mortality
There were no deaths reported during the period from diagnosis to the first outpatient attendance.
Antibody status
Diabetes-associated autoantibody levels were available for 255 cases (85.5%). Of these, 240 (94.1%) had at least one positive antibody. Of the 189 cases with at least three antibodies tested, only five (2.6%) were negative for all antibodies. There were no significant differences between those positive for at least one antibody and those negative for antibodies, with regard to age, sex or degree of acidosis. Again, there were no significant differences between those positive for at least two antibodies and those not, with regard to age, sex or degree of acidosis. Those with a family history of diabetes were no more likely to have positive antibodies.
Type 2 diabetes mellitus
There were 12 cases of type 2 diabetes, six in each year. The annual incidence was 0.72 per 100,000 (95% CI: 0.37–1.26). Type 2 diabetes accounted for 3.8% of all cases and 8.6% of cases aged 10–14 years old. Incidence rates for subpopulations according to age and ethnicity are presented in Table 2.
The female:male ratio was 1:3. Four of the adolescents were European, six were Maori, one was Maori/Pacific Island and one was European/Fijian Indian. The mean age at diagnosis was 13.7 years (range 12.1–14.8 years). Five (42%) had a first-degree relative with type 2 diabetes. In addition, another adolescent had several second-degree relatives with type 2 diabetes. Eight (67%) adolescents were from the Auckland region, two were from the Wellington region, one was from the Bay of Plenty region and one was from the Gisborne region. There were no cases from the South Island.
At diagnosis, three (25%) were not admitted to hospital. The mean blood glucose was 22.4±5.3 mmol/l. Where undertaken (seven cases), pH values were greater than 7.3. Diabetes-associated autoantibody data were available in ten cases. Eight of these ten were negative for all (either three or four) autoantibodies tested, and two were positive for one of four antibodies.
Defining obesity as having a BMI greater than or equal to the 95th percentile for age and sex, 11 (92%) were obese. This compares with the 10.4% of the children and adolescents with type 1 diabetes. The mean BMI was 32.1 kg/m2 (range 21.41–40.35 kg/m2). Acanthosis nigricans was observed in eight (67%) cases. Of the ten children for whom pubertal status was reported, nine were pubertal.
At first follow-up (median 43 days, range 0–132 days), four were treated with an oral hypoglycaemic agent (OHA), five with insulin alone and three with both an OHA and insulin. In the insulin-only group, one had contraindications to OHA use and another two were commenced on an OHA at later follow-up. In the combined treatment group, one was able to discontinue insulin at later follow-up.
Discussion
The incidence of type 1 diabetes in New Zealand children and young adolescents has doubled in the last 20–30 years, with the current annual incidence averaging 17.9 per 100,000 persons at risk. A significant geographical variation in incidence exists, with those living in the North Island being less at risk. This study was able to demonstrate clearly for the first time that this variation is primarily due to the difference in ethnic mix between the two populations, the South Island having a predominant Caucasian population.
In contrast, the second major finding of this study, an emergence of type 2 diabetes in this young population, was only seen in the North Island, where the population has a higher proportion of ethnic minority groups, whose adult populations have high rates of type 2 diabetes.
It is also worth noting the lack of severity of illness at diagnosis, with less than 5% presenting in severe diabetic ketoacidosis. There was no mortality.
The main strength of this study was being able to look at the full spectrum of diabetes mellitus in this age group across the whole country using an established surveillance system which will readily enable follow-up studies for ongoing monitoring. In addition, the surveillance system facilitated a high reporting rate, which resulted in a case ascertainment rate of over 95%. The main limitation of this study was the restriction on the amount of information collected by questionnaire in order to minimise the workload for paediatricians. This meant that information that may have been able to test hypotheses regarding the development of diabetes could not be obtained.
By international standards, the current incidence of type 1 diabetes in young New Zealanders indicates a moderate risk of type 1 diabetes for this population; the lowest risk populations have an incidence of less than one per 100,000, while the highest risk populations have an incidence of greater than 30 per 100,000 [1, 14].
The rate at which the New Zealand incidence has increased is similar to that in many European countries [15]. The international literature is still unclear as to whether the younger age groups are experiencing greater absolute increases in incidence [16]. We cannot add meaningful data to this issue, as the earlier New Zealand studies do not allow comparisons by age group.
Possible reasons for the doubling in incidence fall into the following categories: increased genetic susceptibility of the population, introduction of new or increased exposure to existing environmental trigger(s), and change in epidemiology with a shift in age of onset to a younger population.
Compared with previous New Zealand studies, the demographic characteristics for children developing diabetes are comparable. The age distribution is similar, with children and adolescents of 5 years and older still at significantly increased risk. The sex ratio and geographical variation are also unchanged.
The geographical variation in incidence within New Zealand has been largely explained in this study by examining the effect of ethnicity. Ethnicity, however, is complex in that it is not entirely based on genetics or the environment. Since 1986 the New Zealand census has collected self-identified ethnicity reports [17]. In doing so, the meaning of ethnicity changed from a predominantly racial (genetic) basis to one incorporating sociocultural (environmental) elements of ethnicity [17].
Since there are no previous reliable national incidence data according to ethnicity, this study has been unable to examine trends within and between ethnic groups. Regional Auckland data from 1977 to 1984 reported Maori and Pacific Island rates (5.2 and 6.5 per 100,000, respectively) similar to those found in the current study (5.6 and 9.0, respectively) but a European rate (10.1) less than half the current rate (25.0) [6]. This suggests the following possibilities: (1) a specific genetic susceptibility in the European population to a ubiquitous environmental change; (2) an environmental agent targeting one ethnic population; or (3) increasing genetic susceptibility only in the European population.
The second major finding of this study, the emergence of type 2 diabetes in New Zealand young adolescents, is again in keeping with international trends. The current New Zealand incidence (0.72 per 100,000) is low in comparison to other reported rates: 7.3 and 21 per 100,000 in Japanese school children and Manitoban Indian youths, respectively [18–20]. When considering different subpopulations, however, the incidence in the North Island Maori population is 6.9 per 100,000 for 10- to 14-year-olds, this being more comparable to the rate for 10- to 19-year-olds in Greater Cincinnati in 1994 (7.2/100,000) [21]. The lower rate of type 2 diabetes reported here reflects our lower, yet still alarming, levels of obesity compared with in other populations [22–24]. In addition, this study has only identified incident (recognised and diagnosed) cases.
This study used a national process for data collection, which we have now validated using capture–recapture methodology. It provides good baseline data and suggests a mechanism for regular surveys to document changes in diabetes incidence in New Zealand.
Abbreviations
- NZHIS:
-
New Zealand Health Information Service
- NZPSU:
-
New Zealand Paediatric Surveillance Unit
- OHA:
-
Oral hypoglycaemic agent
References
Onkamo P, Vaananen S, Karvonen M, Tuomilehto J (1999) Worldwide increase in incidence of Type I diabetes—the analysis of the data on published incidence trends. Diabetologia 42:1395–1403
Rosenbloom AL, Joe JR, Young RS, Winter WE (1999) Emerging epidemic of Type 2 diabetes in youth. Diabetes Care 22:345–354
Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J (2000) Incidence of childhood Type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 23:1516–1526
Crossley JR, Upsdell M (1980) The incidence of juvenile diabetes mellitus in New Zealand. Diabetologia 18:29–34
Smith RB (1987) Diabetes in young New Zealanders: results of national survey 1978–82. NZ Med J 100:581–584
Elliott RB, Pilcher CC (1985) Childhood diabetes in the Auckland area. NZ Med J 98:922–923
Mason DR, Scott RS, Darlow B (1987) Epidemiology of insulin-dependent diabetes mellitus in Canterbury, New Zealand. Diabetes Res Clin Pract 3:21–29
Scott RS, Brown LJ, Darlow B, Forbes LV, Moore P (1992) Temporal variation in incidence of IDDM in Canterbury, New Zealand. Diabetes Care 15:895–899
Forbes LV, Brown LJ, Scott R (1993) HLA-DQ beta typing and non-Asp57 alleles in IDDM and nondiabetic subjects in New Zealand. Diabetes Care 16:1179–1183
Forbes LV, Scott RS, Brown LJ, Darlow B (1995) Immunogenetic, clinical, and demographic characterization of childhood Type I diabetes in New Zealand. Diabetes Care 18:1428–1433
Diabetes Epidemiology Research International Group (1988) Geographic patterns of childhood insulin-dependent diabetes mellitus. Diabetes Epidemiology Research International Group. Diabetes 37:1113–1119
LaPorte RE, McCarty D, Bruno G, Tajima N, Baba S (1993) Counting diabetes in the next millennium. Application of capture–recapture technology. Diabetes Care 16:528–534
SAS Institute (1993) SAS Technical report P-243 SAS/STAT software: the genmod procedure. SAS Institute, Cary, NC
Karvonen M, Tuomilehto J, Libman I, LaPorte R (1993) A review of the recent epidemiological data on the worldwide incidence of Type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia 36:883–892
Bingley PJ, Gale EA (1989) Rising incidence of IDDM in Europe. Diabetes Care 12:289–295
Green A, Patterson CC, on behalf of the EURODIAB TIGER Study Group (2001) Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia 44(Suppl 3):B3–B8
New Zealand Health Information Service (2001) Mortality and demographic data 1998. Ministry of Health, Wellington
Kitagawa T, Owada M, Urakami T, Tajima N (1994) Epidemiology of Type 1 (insulin-dependent) and Type 2 (non-insulin-dependent) diabetes mellitus in Japanese children. Diabetes Res Clin Pract 24(Suppl):S7–S13
Kitagawa T, Owada M, Urakami T, Yamauchi K (1998) Increased incidence of non-insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin Pediatr 37:111–115
Dean HJ, Mundy RL, Moffatt M (1992) Non-insulin-dependent diabetes mellitus in Indian children in Manitoba. Can Med Assoc J 147:52–57
Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P (1996) Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 128:608–615
Tyrrell VJ, Richards GE, Hofman P, Gillies GF, Robinson E, Cutfield WS (2001) Obesity in Auckland school children: a comparison of the body mass index and percentage body fat as the diagnostic criterion. Int J Obes Relat Metab Disord 25:164–169
Sigal R, Warram J (1996) The interaction between obesity and diabetes. Curr Opin Endocrinol Diabetes 3:3–9
Young TK, Dean HJ, Flett B, Wood-Steiman P (2000) Childhood obesity in a population at high risk for Type 2 diabetes. J Pediatr 136:365–369
Acknowledgements
A Masonic Child Health Fellowship awarded to P. L. Campbell-Stokes supported this study. We are grateful to the New Zealand Paediatricians and Diabetes Nurse Educators (in particular J. A. Holt, Auckland) who assisted in collection of the data, the NZPSU administration staff, and Sheila Williams for statistical advice. The New Zealand Children’s Diabetes Working Group: Northland: R. Tuck. Auckland: W. Cutfield, P. Hofman. Waikato: D. Bourchier. Taranaki: J. Doran. Bay of Plenty: R. Selby. Hawkes Bay: P. Moore. Manawatu: W. Hunter. Wanganui: J. Goldsmith. Wellington: P. Leadbitter. Nelson-Marlborough: P. McIlroy. Canterbury: B. Darlow. Otago: B. Taylor. Southland: P. Tomlinson.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Campbell-Stokes, P.L., Taylor, B.J. & on behalf of the New Zealand Children’s Diabetes Working Group. Prospective incidence study of diabetes mellitus in New Zealand children aged 0 to 14 years. Diabetologia 48, 643–648 (2005). https://doi.org/10.1007/s00125-005-1697-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1697-3