Abstract
Aims/hypothesis
Epidemiological research indicates that long-chain n-3 polyunsaturated fatty acids (LC n-3 PUFA) improve insulin resistance. The aim of this study was to investigate the effects of seafood consumption on insulin resistance in overweight participants during energy restriction.
Methods
In this 8 week dietary intervention, 324 participants (20–40 years, BMI 27.5–32.5 kg/m2, from Iceland, Spain and Ireland) were randomised by computer to one of four energy-restricted diets (−30E%) of identical macronutrient composition but different LC n-3 PUFA content: control (n = 80; no seafood; single-blinded); lean fish (n = 80; 150 g cod, three times/week); fatty fish (n = 84; 150 g salmon, three times/week); (4) fish oil (n = 80; daily docosahexaenoic/eicosapentaenoic acid capsules, no other seafood; single-blinded). Fasting glucose, insulin, adiponectin, plasma triacylglycerol and fatty acids in erythrocyte membrane were measured at baseline and endpoint. Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR). Linear models with fixed effects and covariates were used to investigate the effects of seafood consumption on fasting insulin and HOMA-IR at endpoint in comparison with the control group.
Results
Of the participants, 278 (86%) completed the intervention. Fish oil intake was a significant predictor of fasting insulin and insulin resistance after 8 weeks, and this finding remained significant even after including weight loss, triacylglycerol reduction, increased LC n-3 PUFA in membranes or adiponectin changes as covariates in the statistical analysis. Weight loss was also a significant predictor of improvements.
Conclusions/interpretation
LC n-3 PUFA consumption during energy reduction exerts positive effects on insulin resistance in young overweight individuals, independently from changes in body weight, triacylglycerol, erythrocyte membrane or adiponectin.
Trial registration: ClinicalTrials.gov NCT00315770.
Funding: The YOUNG study is part of the SEAFOODplus Integrated Project, which is funded by the EC through the 6th Framework Programme Contract No. FOOD-CT-2004-506359.
Similar content being viewed by others
Introduction
Obesity and type 2 diabetes mellitus are characterised by impaired insulin-stimulated glucose disposal in skeletal muscle [1]. Fish oil (and its important long-chain n-3 polyunsaturated fatty acids (LC n-3 PUFA) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) protect against insulin resistance and obesity in rodents fed high-fat diets, and might reduce insulin response to glucose in healthy humans [2–4]. According to epidemiological studies, fish consumption is inversely related to 2 h glucose levels during follow-up, and is associated with a reduced risk of developing impaired glucose tolerance in the elderly [5, 6]. An ecological study indicates that the prevalence of type 2 diabetes in men in the Nordic countries is significantly and inversely associated with both the n-3 PUFA and EPA content of milk, and positively associated with the ratio of n-6/n-3 fatty acids in milk [7]. According to a meta-analysis of published clinical trials, significant dose–response effects of EPA on HbA1c and triacylglycerol, and of DHA on fasting blood glucose levels, HbA1c and triacylglycerol, have been demonstrated in individuals with type 2 diabetes [8]. However, several intervention studies concerning LC n-3 PUFA supplementation and insulin resistance have reported negative outcomes, which require further clarification [9].
The mechanism responsible for the possible fish oil-induced prevention of insulin resistance is unclear, but different studies have demonstrated a strong association between elevated triacylglycerol concentration (plasma and/or tissues) and insulin resistance [10]. In animal models, insulin resistance is significantly correlated with hepatic or plasma triacylglycerol content [11, 12]. Fish oil intake has been shown to decrease plasma and liver triacylglycerol levels and VLDL-triacylglycerol secretion, and to suppress postprandial hypertriacylglycerolaemia [13].
Increasing evidence also suggests that the fatty acid composition of membrane phospholipids of insulin target tissues, such as liver, fat pad and skeletal muscle, is a critical factor that influences both insulin secretion and its biological actions (e.g. changes in membrane fluidity, diacylglycerol second messenger function) [14, 15]. The fatty acid profile of muscle membrane phospholipids has been associated with insulin sensitivity in rodents [16, 17] and humans [18–21]. These studies demonstrated a positive correlation between a high content of LC n-3 PUFA and insulin sensitivity.
Adiponectin is an adipocyte-derived hormone that stimulates glucose utilisation and fatty acid oxidation in muscle and decreases hepatic gluconeogenesis, and a low plasma level of this protein is an independent risk factor for the future development of type 2 diabetes [22]. Moreover, an association between circulating adiponectin and plasma n-3 PUFA was recently found in healthy humans [23]. Based on this evidence, it has been hypothesised that possible protective effects of EPA and DHA may involve induction of adiponectin. In animal research, the intake of diets rich in EPA and DHA (5.3% of total energy intake) leads to elevated systemic concentrations of adiponectin, largely independent of food intake or adiposity [24].
To investigate the effects of the consumption of seafood containing different amounts of LC n-3 PUFA on insulin resistance, we conducted a randomised controlled dietary intervention trial in overweight and obese young adults from three European countries. The goals of the present study were to investigate whether seafood intake during energy restriction improves fasting insulin and insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR]). We also paid particular attention to plasma triacylglycerol, fatty acid composition of membrane phospholipids, and plasma adiponectin levels, to estimate their combined or independent contribution to changes in insulin sensitivity during this 8 week trial.
Methods
Participants
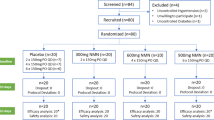
A total of 324 overweight individuals (138 men, 186 women) were recruited to our study SEAFOODplus YOUNG (http://www.seafoodplus.org, accessed 4 April 2008) through advertisements on hospital and university noticeboards, 140 from Iceland, 120 from Spain and 64 from Ireland. All subjects were screened for inclusion and exclusion criteria. The inclusion criteria were a BMI in the range of 27.5–32.5 kg/m2, age 20–40 years and a waist circumference of ≥94 cm and ≥80 cm for men and women, respectively. Exclusion criteria were weight change (±3 kg) in response to a weight loss diet within 3 months before the start of the study, use of supplements containing n-3 fatty acids, calcium or vitamin D during the last 3 months, allergy to fish, pregnancy or lactation, drug treatment for hypertension, hyperlipidaemia and diabetes mellitus. None of the participants was diagnosed with type 2 diabetes, but 12 participants had impaired fasting glycaemia (>5.6 and <6.9 mmol/l). About 86% (n = 278) of the participants completed the intervention. The study was approved by the National Bioethical Committee in Iceland (04–031), the Ethical Committee of the University of Navarra in Spain (24/2004) and the Clinical Research Ethics Committee of the Cork University Hospital in Ireland. The study followed the Helsinki guidelines (as revised in 2000), and all participants gave their written consent. Power calculations for the study were based on weight loss, the primary endpoint of the study. It was estimated that a participation rate of 70–80% allowed the detection of approximately 1 kg difference in weight loss between the four diet groups, assuming an sSD of 3 kg, a p value for significance of <0.05 and a statistical power of >0.8.
Study design
This study was a randomised controlled dietary intervention trial. It was conducted at the Landspitali-University Hospital in Reykjavik, Iceland, the University College of Cork, Ireland, and the University of Navarra in Pamplona, Spain, and took place during the period from April 2004 to November 2005. The intervention lasted for 8 consecutive weeks, during which time the participants were instructed to follow a diet restricted in terms of energy to 70% of their normal intake, which was determined by calculating energy expenditure by Harris–Benedict equations [25], using a correction factor to take into account the overweight status of the subjects [26] and an adjustment for physical activity level [27].
The participants were randomly assigned to one of the following four diets, which varied with respect to the amount of LC n-3 PUFA:
-
Diet 1:
no seafood (control; 6×500 mg sunflower oil capsules/day, Loders Croklaan [Lipid Nutrition], Wormerveer, the Netherlands; encapsulated by Banner Pharmacaps, Tilburg, the Netherlands);
-
Diet 2:
lean fish (150 g cod, three times/week);
-
Diet 3:
fatty fish (150 g salmon, three times/week); or
-
Diet 4:
fish oil capsules (6× 500 mg capsules/day, Loders Croklaan, encapsulated by Banner Pharmacaps).
A chart of participant flow through the study is presented in Electronic supplementary material Fig. 1.
Diet group 1 and 4 were single-blinded. For comparability, the diets prescribed were of identical macronutrient composition: total fat (~30% of total energy), carbohydrate (~50% of total energy), protein (~20% of total energy) and dietary fibre (~20–25 g). Each diet provided different amounts of LC n-3 PUFA: the placebo capsules in diet group 1 provided 0 g/day, cod in diet 2 provided 0.26 g/day, salmon in diet group 3 provided 2.1 g/day, and fish oil capsules provided 1.3 g/day. Each subject received a detailed meal plan to follow, as well as recipe booklets and instructions, so as to minimise differences between diets in terms of sources of fat (other than LC n-3 PUFA), fruit and vegetable consumption and meal frequency. The physical activity level of the participants remained unchanged during the intervention. (For more information on the intervention see Thorsdottir et al. [28].) To measure compliance, seafood intake was assessed by a validated food frequency questionnaire (FFQ) and dietary intake was assessed by 2 day weighed food records before baseline (habitual diet) and in week 6 or later of the intervention trial [29]. Compliance was also assessed by analysing n-3 and n-6 fatty acids in erythrocyte phospholipids in fasting blood samples, as previously reported [28]. Results showed good compliance with the intervention diets.
Anthropometric measurements
All measurements were done using standard procedures, as outlined in a research protocol approved and used by all centres participating in the study. Anthropometrical measurements were performed at baseline and endpoint of the study. Body weight was measured in light underwear on a calibrated scale (model no. 708; Seca, Hamburg, Germany). The height of the subjects was measured with a calibrated stadiometer. Fat mass and fat-free mass were assessed by bioelectrical impedance analysis (BIA; Bodystat 1500; Bodystat, Douglas, Isle of Man, British Isles).
Biochemical measurements
Participants were instructed to avoid strenuous exercise and alcohol consumption the day before the drawing of blood samples at baseline and endpoint, which were analysed for fasting concentrations of blood glucose (mmol/l), insulin (pmol/l), plasma triacylglycerol (mmol/l) and adiponectin (μg/ml). All the blood analyses were performed centrally. Insulin was measured with electrochemiluminescence immunoassay (ECLIA) on a Modular Analytics E170 system from Roche Diagnostics (Manheim, Germany). Plasma triacylglycerol and glucose were analysed using an enzymatic colorimetric assay and an automated analyzer (Hitachi 911; Roche Diagnostics). Adiponectin was measured with a radioimmunoassay kit developed by Linco Research (St Charles, MO, USA). Fatty acid composition in extracted erythrocyte membrane phospholipids was analysed by gas chromatography under the conditions described previously [30].
Insulin resistance
In the present study we used the updated HOMA model (known as HOMA2, computer model) to calculate insulin resistance (referred to as HOMA-IR), as described previously [31], which can be downloaded as a calculator or Excel spread sheet from the homepage of the Diabetes Trials Unit, University of Oxford, UK (http://www.dtu.ox.ac.uk/homa, accessed 4 April 2008).
Statistical analysis
The data were entered into the SPSS statistical package, version 11.0 (SPSS, Chicago, IL, USA). Wilcoxon test was used to calculate whether there were significant changes in the variables between baseline and endpoint. The baseline characteristics of the groups were compared using linear models with fixed effects (country, sex, diet group) and covariate (age). To find out whether diet groups predict endpoint fasting insulin and HOMA-IR after 8 weeks, linear models with fixed effects (country, sex, diet group) and covariates (age, baseline value of the relevant outcome variable) were constructed. To find out whether changes in body weight, triacylglycerol, membrane lipid fatty acids or adiponectin can explain possible effects of diet groups on outcomes, the blood biochemical and anthropometrical variables were entered as additional covariates in separate linear models. Insulin and HOMA-IR values were log-transformed for this analysis. Results from the linear models are shown as parameter estimates where the cod, salmon and fish oil groups were compared separately with the control group. The numbers in the parameter estimates (B, lower confidence limit, higher confidence limit) were back-transformed and are shown as 1 – B, 1 – lower confidence limit, 1 – higher confidence limit, respectively, thus giving percentage differences between groups with respect to endpoint variables. A p value of less than 0.05 was regarded as statistically significant.
Results
Baseline anthropometric variables, hormones, HOMA-IR and fatty acid values are reported in Table 1. Despite randomisation, the cod group had higher triacylglycerol levels at baseline than the control group (p = 0.017), and all intervention groups had lower EPA values at baseline than the control group (p = 0.004–0.026). Other baseline parameters did not significantly differ between groups. During the intervention, the body weight of participants decreased (−5.2 ± 3.2 kg, p < 0.001, n = 278), as did fasting insulin, glucose, triacylglycerol and adiponectin levels and HOMA-IR, whereas LC n-3 PUFA content in membrane lipids significantly increased (all p < 0.001; Table 2).
Linear models
The following variables were entered into the linear models to find out whether diet groups can predict endpoint fasting insulin (Table 3) and HOMA IR (Table 4): sex, country, diet groups, age and baseline value of the dependent variable. Fasting insulin and HOMA-IR were significantly lower in the fish oil group than in the control group at endpoint (16.4%, p = 0.025 and 17.2%, p = 0.022, respectively). To find out whether the effects of diet groups on endpoint fasting insulin and HOMA-IR can be explained by changes in weight loss, triacylglycerol reduction, increased EPA/DHA content in membrane lipids or adiponectin, these anthropometric and blood chemical variables were entered as additional covariates. The differences between the fish oil group and control remained significant (values 15.8% and 16.1% lower than control, respectively), and weight loss was revealed to be an additional significant predictor of both outcomes each kg weight loss predicted a 3.4% and 3.5% reduction, respectively). Triacylglycerol reduction, EPA changes and adiponectin changes did not contribute significantly in the linear models. The results of the linear models show estimated changes in endpoint insulin (Table 3) and HOMA-IR (Table 4) in response to an increase in EPA; the use of DHA instead did not change the results.
Discussion
In this randomised dietary intervention trial we investigated the effects of seafood intake and weight loss on fasting insulin and HOMA-IR in young overweight and obese European adults from three different countries following an energy-restricted diet. Participants consumed cod (150 g, three times/week), salmon (150 g, three times/week), fish oil (6 × 500 mg capsules/day) or placebo capsules (6 × 500 mg capsules/day), but otherwise consumed diets of identical macronutrient composition and percentage energy restriction [28]. During these 8 weeks, average fasting insulin decreased and HOMA-IR improved in the participants.
The most important finding of our study is that the fish oil diet reduces fasting insulin and improves HOMA-IR to a significantly greater extent than the control diet (between 15.8% and 17.2%, depending on the statistical model) and to an extent similar to that observed with a weight loss of 4.7 kg (calculated by dividing the B coefficients of the fish oil group and weight loss from the linear models). Animal research, ecological and epidemiological studies have shown associations between LC n-3 PUFA intake and insulin sensitivity [2, 5, 6, 7], but, according to reviews [9, 32], there is relatively little evidence from interventions that n-3 PUFA supplementation has positive effects on insulin sensitivity in humans. An early experimental study reported positive effects of 3 g/day (in total) of EPA and DHA on insulin sensitivity (measured by in vivo insulin-stimulated glucose uptake by simultaneous infusions of glucose and insulin) in six individuals with type 2 diabetes [33]. Another study [34], which investigated the combined effects of fish consumption and weight loss on cardiovascular risk factors in 69 overweight patients, found that the greatest decreases in fasting insulin and glucose occurred in the fish + weight-loss group. However, there were no independent effects of fish on glucose or insulin. A similar intervention study involving 116 overweight insulin-resistant women showed independent (from weight loss) effects of LC n-3 PUFA supplementation on triacylglycerol and adiponectin levels, but not insulin sensitivity [35]. The Kuopio, Aarhus, Naples, Wollongong and Uppsala (KANWU) study, a controlled multi-centre isoenergetic dietary intervention study focusing on dietary fat composition but not weight loss in 162 individuals, showed that decreasing dietary saturated fatty acid intake and increasing monounsaturated fatty acid intake improves insulin sensitivity, but no effect of fish oil supplementation on insulin sensitivity was found, despite reduced plasma triacylglycerol concentrations [36]. Similarly, a recent study investigating 29 Indian Asians, a group particularly susceptible to the metabolic syndrome and type 2 diabetes, did not find an effect of LC n-3 PUFA supplementation on insulin resistance, even though supplementation was associated with improved fasting and postprandial plasma triacylglycerol metabolism [37]. In comparison with the above-mentioned studies, the present study investigated a greater number of subjects. The higher statistical power can explain our significant findings. Significant weight loss was achieved in our study, and it can be speculated that LC n-3 PUFA exert their positive health effects on insulin sensitivity best during weight loss; however, similar or greater amounts of weight loss were achieved in similar studies [34, 35] that did not report significant effects of LC n-3 PUFA on insulin sensitivity.
We cannot explain why endpoint fasting insulin and HOMA-IR values for the salmon group were not significantly different from those for the control group, despite the fact that salmon provided the highest dose of LC n-3 PUFA in our study. The estimated improvements for the salmon group were lower than those for the fish oil group (7.5–10.5%), but were not significantly different (p = 0.191–0.334, analyses not shown). The LC n-3 PUFA from salmon were bioavailable, as indicated by increases in DHA and EPA in erythrocyte membrane. It can be speculated that other constituents in salmon could counteract the effects of LC n-3 PUFA. However, it should be mentioned that predicted changes in the salmon group were the same direction as those in the fish oil group, but they did not reach statistical significance. This is in contrast to the cod group, which did not show any effects compared with the control group.
Unexpectedly, the positive effects of LC n-3 PUFA on the outcome variables during the 8 week intervention cannot be explained by reductions in weight loss or triacylglycerol, or changes in membrane fatty acids or adiponectin. It has long been recognised that fish oil can decrease plasma and liver triacylglycerol levels and VLDL-triacylglycerol secretion, and suppress postprandial hypertriacylglycerolaemia [13]. However, the inclusion of plasma triacylglycerol in our linear models did not alter the strength of fish oil as a significant predictor of insulin sensitivity.
In several cross-sectional studies, measures of insulin sensitivity are negatively correlated with the percentage of individual long-chain polyunsaturated fatty acids in the phospholipid fraction of muscle in patients or healthy subjects [18, 19]. An animal study investigated the effects of fish oil intake on membrane composition and insulin sensitivity in rats and showed that incorporation of n-3 fatty acids into adipocyte membrane phospholipids was higher in rats fed fish oil than in the control group, and insulin action was positively correlated with the fatty acid unsaturation index in membrane phospholipids [38]. Furthermore, a recent human intervention study showed that increased incorporation of LC n-3 PUFA and reduced saturated fatty acids in skeletal muscle membrane phospholipids is associated with better insulin sensitivity [21]. According to our linear models, changes in membrane fatty acid composition do not play an independent role in the improvements in insulin sensitivity in our participants. It is possible that phospholipid composition in erythrocytes does not reflect phospholipid composition in muscle [39] and/or the study duration of 8 weeks was too short to allow a full steady state to be achieved with respect to phospholipid fatty acid composition of skeletal muscle, liver and depot fat.
Adiponectin is a hormone secreted by the adipose tissue, and its administration enhances insulin action in animals [40]. Cross-sectional studies have shown adiponectin levels to be associated with insulin sensitivity [41, 42]. When we use our baseline data to calculate correlations between insulin sensitivity and adiponectin (results not shown), we see the same associations as in the aforementioned cross-sectional studies. However, according to the fitted statistical models, changes in adiponectin did not predict changes in insulin sensitivity. It could be that during the intervention, other factors, namely fish oil intake and weight loss, had a much stronger effect on insulin sensitivity then the small changes in adiponectin, such that effects of adiponectin were not visible in our analysis.
Limitations
A common limitation of a dietary intervention trial is blinding. In our study the fish oil and control groups were single-blinded, whereas the cod and salmon groups were not (fish was received as a fillet). Another limitation is the uncertainty of whether the dietary intakes during the study period were as prescribed. This risk was minimised by the intense support provided by the staff and frequent contact with the participants via phone and personal visits. Compliance was tested during the intervention trial using an FFQ, validated for assessing frequency of seafood consumption [29], at the endpoint of the study, and measuring erythrocyte fatty acid composition at the end of the intervention. Changes in n-3 fatty acids in erythrocyte membrane were in accordance with fatty acid composition of the diets, and results from the FFQ confirmed good compliance.
Conclusion
Fish oil consumption during an 8 week energy-restricted diet exerts positive effects on fasting insulin and on a measure of insulin resistance in young overweight or obese individuals. These effects are independent from weight loss, changes in plasma triacylglycerol, erythrocyte membrane EPA/DHA or adiponectin concentrations. Weight loss is an additional independent predictor of improvements in individuals who consume fish oils. The present randomised dietary intervention trial supports previous epidemiological evidence, and demonstrates the importance of LC n-3 PUFA consumption for improvement of insulin resistance and, possibly, for the prevention of type 2 diabetes in addition to weight-lowering strategies. Future intervention studies are required to confirm the results of this study.
Abbreviations
- DHA:
-
docosahexaenoic acid
- EPA:
-
eicosapentaenoic acid
- FFQ:
-
food frequency questionnaire
- HOMA-IR:
-
homeostasis model assessment of insulin resistance
- LC n-3 PUFA:
-
long-chain n-3 polyunsaturated fatty acids
References
Walker M (1995) Obesity, insulin resistance, and its link to non-insulin-dependent diabetes mellitus. Metabolism 44:18–20
Perez-Matute P, Perez-Echarri N, Martinez JA, Marti A, Moreno-Aliaga MJ (2007) Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-alpha. Br J Nutr 97:389–398
Ruxton CH, Reed SC, Simpson MJ, Millington KJ (2004) The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 17:449–459
Delarue J, LeFoll C, Corporeau C, Lucas D (2004) N-3 long chain polyunsaturated fatty acids: a nutritional tool to prevent insulin resistance associated to type 2 diabetes and obesity? Reprod Nutr Dev 44:289–299
Feskens EJ, Loeber JG, Kromhout D (1994) Diet and physical activity as determinants of hyperinsulinemia: the Zutphen Elderly Study. Am J Epidemiol 140:350–360
Feskens EJ, Bowles CH, Kromhout D (1991) Inverse association between fish intake and risk of glucose intolerance in normoglycemic elderly men and women. Diabetes Care 14:935–941
Thorsdottir I, Hill J, Ramel A (2004) Omega-3 fatty acid supply from milk associates with lower type 2 diabetes in men and coronary heart disease in women. Prev Med 39:630–634
Friedberg CE, Janssen MJ, Heine RJ, Grobbee DE (1998) Fish oil and glycemic control in diabetes. A meta-analysis. Diabetes Care 21:494–500
Roche HM (2005) Fatty acids and the metabolic syndrome. Proc Nutr Soc 64:23–29
Lombardo YB, Chicco AG (2006) Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J Nutr Biochem 17:1–13
Pagliassotti MJ, Prach PA, Koppenhafer TA, Pan DA (1996) Changes in insulin action, triglycerides, and lipid composition during sucrose feeding in rats. Am J Physiol 271:R1319–R1326
Thorburn AW, Storlien LH, Jenkins AB, Khouri S, Kraegen EW (1989) Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr 49:1155–1163
Herzberg GR, Rogerson M (1988) Hepatic fatty acid synthesis and triglyceride secretion in rats fed fructose- or glucose-based diets containing corn oil, tallow or marine oil. J Nutr 118:1061–1067
Clamp AG, Ladha S, Clark DC, Grimble RF, Lund EK (1997) The influence of dietary lipids on the composition and membrane fluidity of rat hepatocyte plasma membrane. Lipids 32:179–184
Storlien LH, Pan DA, Kriketos AD et al (1996) Skeletal muscle membrane lipids and insulin resistance. Lipids 31:S261–265
Ayre KJ, Phinney SD, Tang AB, Stern JS (1998) Exercise training reduces skeletal muscle membrane arachidonate in the obese (fa/fa) Zucker rat. J Appl Physiol 85:1898–1902
Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW (1991) Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 40:280–289
Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV (1993) The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 328:238–244
Pan DA, Lillioja S, Milner MR et al (1995) Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J Clin Invest 96:2802–2808
Vessby B, Tengblad S, Lithell H (1994) Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 37:1044–1050
Haugaard SB, Madsbad S, Høy CE, Vaag A (2006) Dietary intervention increases n-3 long-chain polyunsaturated fatty acids in skeletal muscle membrane phospholipids of obese subjects. Implications for insulin sensitivity. Clin Endocrinol (Oxf) 64:169–178
Lindsay RS, Funahashi T, Hanson RL et al (2002) Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 360:57–58
Fernandez-Real JM, Vendrell J, Ricart W (2005) Circulating adiponectin and plasma fatty acid profile. Clin Chem 51:603–609
Flachs P, Mohamed-Ali V, Horakova O et al (2006) Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia 49:394–397
Cankayali I, Demirag K, Kocabas S, Resat Moral A (2004) The effects of standard and branched chain amino acid enriched solutions on thermogenesis and energy expenditure in unconscious intensive care patients. Clin Nutr 23:257–63
Salvino RM, Dechicco RS, Seidner DL (2004) Perioperative nutrition support: who and how. Cleve Clin J Med 71:345–351
Nordic Council of Ministers (2004) Nordic Nutrition Recommendations 2004. Integrating nutrition and physical activity, 4th edn. Nordic Council of Ministers, Copenhagen
Thorsdottir I, Tomasson H, Gunnarsdottir I et al (2007) Randomized trial of weight-loss diets for young adults varying in fish and fish oil content. Int J Obesity 31:1560–1566
Birgisdottir BE, Kiely M, Martinez JA, Thorsdottir I (2008) Validity of a food frequency questionnaire to assess intake of seafood in adults in three European countries. Food Control 19:648–653
Bandarra NM, Palma P, Batista I et al (2002) Effect of a supplemented diet with canned sardine on the lipid fraction of human plasma and erythrocytes. J Aquat Food Prod Tech 11:177–185
Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495
Vessby B (2000) Dietary fat and insulin action in humans. Br J Nutr 83:S91–S96
Popp-Snijders C, Schouten JA, Heine RJ, van der Meer J, van der Veen EA (1987) Dietary supplementation of omega-3 polyunsaturated fatty acids improves insulin sensitivity in non-insulin-dependent diabetes. Diabetes Res 4:141–147
Mori TA, Bao DQ, Burke V, Puddey IB, Watts GF, Beilin LJ (1999) Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr 70:817–825
Krebs JD, Browning LM, McLean NK et al (2006) Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond) 30:1535–1544
Vessby B, Unsitupa M, Hermansen K et al (2001) Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia 44:312–319
Brady LM, Lovegrove SS, Lesauvage SV et al (2004) Increased n-6 polyunsaturated fatty acids do not attenuate the effects of long-chain n-3 polyunsaturated fatty acids on insulin sensitivity or triacylglycerol reduction in Indian Asians. Am J Clin Nutr 79:983–991
Luo J, Rizkalla SW, Boillot J et al (1996) Dietary (n-3) polyunsaturated fatty acids improve adipocyte insulin action and glucose metabolism in insulin-resistant rats: relation to membrane fatty acids. J Nutr 126:1951–1958
Di Marino L (2000) Is the erythrocyte membrane fatty acid composition a valid index of skeletal muscle membrane fatty acid composition? Metabolism 49:1164–1166
Havel PJ (2002) Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 13:51–59
Cnop M, Havel PJ, Utzschneider KM et al (2003) Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46:459–469
Tschritter O, Fritsche A, Thamer C et al (2003) Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 52:239–243
Acknowledgements
This work is included in the SEAFOODplus YOUNG, co-ordinated by Prof. Inga Thorsdottir, being part of the SEAFOODplus Integrated Project, which is funded by the European Commission through the 6th Framework Programme Contract with Ref. FOOD-CT-2004–506359. Thanks are given to the EU Commission for financial support, and to the volunteers who participated in the study.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM Fig. 1
Chart of participant flow through the study (PDF 13.2 KB)
Rights and permissions
About this article
Cite this article
Ramel, A., Martinéz, A., Kiely, M. et al. Beneficial effects of long-chain n-3 fatty acids included in an energy-restricted diet on insulin resistance in overweight and obese European young adults. Diabetologia 51, 1261–1268 (2008). https://doi.org/10.1007/s00125-008-1035-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-008-1035-7