Abstract
Aims/hypothesis
Protein kinase C (PKC) regulates exocytosis in various secretory cells. Here we studied intracellular translocation of the PKC isoenzymes PKCα and PKCδ, and investigated how activation of PKC influences glucagon secretion in mouse and human pancreatic alpha cells.
Methods
Glucagon release from intact islets was measured in static incubations, and the amounts released were determined by RIA. Exocytosis was monitored as increases in membrane capacitance using the patch-clamp technique. The expression of genes encoding PKC isoforms was analysed by real-time PCR. Intracellular PKC distribution was assessed by confocal microscopy.
Results
The PKC activator phorbol 12-myristate 13-acetate (PMA) stimulated glucagon secretion from mouse and human islets about fivefold (p < 0.01). This stimulation was abolished by the PKC inhibitor bisindolylmaleimide (BIM). Whereas PMA potentiated exocytosis more than threefold (p < 0.001), BIM inhibited alpha cell exocytosis by 60% (p < 0.05). In mouse islets, the PKC isoenzymes, PKCα and PKCβ1, were highly abundant, while in human islets PKCη, PKCε and PKCζ were the dominant variants. PMA stimulation of human alpha cells correlated with the translocation of PKCα and PKCδ from the cytosol to the cell periphery. In the mouse alpha cells, PKCδ was similarly affected by PMA, whereas PKCα was already present at the cell membrane in the absence of PMA. This association of PKCα in alpha cells was principally dependent on Ca2+ influx through the L-type Ca2+ channel.
Conclusions/interpretation
PKC activation augments glucagon secretion in mouse and human alpha cells. This effect involves translocation of PKCα and PKCδ to the plasma membrane, culminating in increased Ca2+-dependent exocytosis. In addition, we demonstrated that PKCα translocation and exocytosis exhibit differential Ca2+ channel dependence.
Similar content being viewed by others
Introduction
Glucagon is produced by the pancreatic alpha cells and released in response to decreased blood glucose. It is the most important hyperglycaemic hormone in animals and man [1, 2]. Glucagon increases plasma glucose by promoting hepatic glycogenolysis. In type 2 diabetes, the normal balance between glucagon and insulin is disturbed [3]. Collectively, these defects culminate in increased hepatic glucose production [4]. Because of its important role in hyperglycaemia in type 2 diabetes, glucagon has become a very attractive target for diabetes research.
Glucagon secretion is regulated through paracrine, metabolic and hormonal/neuronal mechanisms [5]. The latter includes adrenaline (epinephrine) [6, 7], glucagon-like peptide 1 [8], somatostatin [9] and glucagon itself [10]. Like the neighbouring beta and delta cells [11], alpha cells are electrically excitable and fire action potentials at low glucose concentrations or when glucagon secretion is stimulated by amino acids like arginine [12]. These action potentials result from the opening of voltage-gated K+, Na+ and Ca2+ channels [13]. We have previously demonstrated that glucagon secretion can be regulated by a KATP channel-dependent mechanism in rodent and human alpha cells [14]. Electrical activity is possible in a narrow window of low KATP channel activity, resulting in partial depolarisation, which triggers opening of voltage-gated Na+ and Ca2+ channels. Increasing glucose levels have been postulated to mediate the closure of remaining active KATP channels, thereby leading to stronger membrane depolarisation. This in turn causes voltage-dependent inactivation of the membrane conductance involved in action potential firing, culminating in inhibition of glucagon secretion and alpha cell exocytosis. In rodent alpha cells, exocytosis is Ca2+-dependent [13] and basal glucagon secretion principally depends on influx through N-type Ca2+ channels. In addition, alpha cells are equipped with L-type Ca2+ channels, which are important for glucagon secretion occurring under conditions associated with elevation of intracellular cyclic AMP (like in the presence of adrenaline) [6].
Glucagon secretion is also modulated by protein kinase C (PKC) [15, 16]. Genetic screening has unveiled ten distinct PKC isotype genes [17]. The PKC superfamily has been subdivided on the basis of properties: conventional/classical PKCs (α, βI, βII and γ), novel PKCs (η, ε, δ and θ) and atypical PKCs (ι/λ and ζ). Activation of PKC by exogenous diacylglycerol [18, 19] and phorbol esters augments insulin [20] and glucagon secretion in rat [15]. Conversely, pharmacological inhibitors of PKC suppress glucose-induced insulin secretion [21, 22].
Several PKC isoenzymes have been shown to be implicated in control of exocytosis [23–26], including PKCα [27] and PKCδ [28], but the effect of PKC on exocytosis in pancreatic alpha cells remains to be established. In rat beta cells, the activation of several PKC isoforms leads to their translocation from the cytosol to the cell membrane [29]. Conventional PKCs are activated by binding to Ca2+ and diacylglycerol, whereas activation of novel PKCs is only sensitive to diacylglycerol [17]. This may explain why PKCα, but not PKCδ becomes translocated to the plasma membrane in beta cells in response to the glucose-induced elevation of [Ca2+]i [27, 29]. Once at the plasma membrane, PKC probably acts by phosphorylating downstream target proteins. One potential PKC substrate is mammalian homologue of Unc-18 (Munc-18). In many cell types, Munc-18 is crucial for the docking and priming of secretory granules, as well as for actual fusion with the plasma membrane [30]. It has been demonstrated that Munc-18 is phosphorylated by PKC in response to phorbol ester treatment [31, 32].
Here we studied PKC-dependent exocytosis and glucagon secretion in mouse and human alpha cells. We also investigated islet level expression of genes encoding PKC isoenzymes as well as the redistribution of the two isoenzymes, PKCα and PKCδ, in response to phorbol 12-myristate 13-acetate (PMA) and Ca2+ influx.
Methods
Tissues and cell culture
Islets from NMRI mice were isolated by collagenase digestion. All experimental procedures performed on mice were approved by the Lund-Malmö Ethics Committee. Islets were incubated in Ca2+-free buffer to obtain a suspension of individual cells. Two days prior to experiments, these cells were plated on plastic Petri dishes (for patch-clamp recording) or glass cover slips (for confocal microscopy) and maintained in tissue culture medium for 6 h. The culture medium consisted of RPMI 1640 supplemented with 10% (vol./vol.) fetal calf serum, 100 IU/ml penicillin and 10 μg/ml streptomycin. Human islets were from non-diabetic individuals (BMI 17.6–29.0 kg/m2, aged 26–73 years) and provided by the Nordic network for clinical islets transplantation (O. Korsgren, Uppsala University, Sweden). The human islets were cultured at 37°C (5% CO2) for 1 to 9 days prior to the experiments in CMRL 1066 (ICN Biomedicals, Costa Mesa, CA, USA) supplemented with 10 mmol/l HEPES, 2 mmol/l l-glutamine, 50 μg/ml gentamicin, 0.25 μg/ml fungizone (Gibco, BRL, Gaithersburg, MD, USA), 20 μg/ml ciprofloxacin (Bayer Healthcare, Leverkusen, Germany) and 10 mmol/l nicotinamide. All procedures were approved by the ethical committees at Uppsala and Lund Universities.
In vitro pancreatic islet glucagon release
Freshly isolated islets were pre-incubated for 30 min at 37°C in a KRB buffer (pH 7.4) consisting of (mmol/l) 120 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4, 2.5 CaCl2, 1.2 KH2PO4, 1 glucose, 10 HEPES at pH 7.4 and 1 mg/ml BSA. The medium was gassed with 95% O2 and 5% CO2 to obtain constant pH and oxygenation. Groups of twelve islets were incubated for 60 min at 37°C in 1 ml KRB buffer supplemented as indicated. PMA and bisindolylmaleimide (BIM) were purchased from Calbiochem (San Diego, CA, USA). Immediately after incubation, an aliquot of the medium was removed to determine glucagon content as previously described [14].
Capacitance measurement
Experiments were carried out on mouse alpha cells as detailed elsewhere [10]. Whole-cell currents and exocytosis were recorded using an EPC-9 patch-clamp amplifier and Pulse software (version 8.50) (both Heka Electronics, Lambrecht, Germany). Mouse alpha cells were identified by their small size and Na+-current inactivation properties [13, 14]. The extracellular medium contained (mmol/l) 118 NaCl, 20 tetraethylammonium chloride, 5.6 KCl, 2.6 CaCl2, 1.2 MgCl2, 5 HEPES (pH 7.4 with NaOH) and 5 glucose. The electrophysiological measurements were conducted using the perforated patch technique and pipette solution containing (mmol/l) 76 Cs2SO4, 10 NaCl, 10 KCl, 1 MgCl2 and 5 HEPES (pH 7.35 with CsOH), and 60 µg/ml amphotericin B. Exocytosis was monitored as changes in membrane capacitance using the software-based lock-in function of the Pulse software. Experiments were conducted at 32 to 34°C.
Relative quantitative real-time PCR
Human and mouse pancreatic islets were dissolved in TRIzol (Invitrogen, Carlsbad, CA, USA) and stored at −80°C. Total islet RNA was extracted according to a modified TRIzol protocol and reverse-transcribed into cDNA using TaqMan Reverse transcription (Applied Biosystems, Foster City, CA, USA) as described elsewhere [33]. Relative quantitative real-time PCR of human and mouse PKC genes was performed using a kit and primer assays (QuantiFast SYBR Green PCR and QuantiTect; Qiagen, Venlo, the Netherlands) according to the manufacturer’s instructions. The expression of genes encoding pancreatic islet PKC was calculated relative to the housekeeping gene GAPDH using the ∆∆Ct method [34].
Immunohistochemistry
Mouse and human islets were dissociated into single cells, plated on glass cover slips and cultured overnight. The culture media were replaced by the same KRB buffer as in the secretion assay during 30 min of pre-incubation. The cells were then incubated for 1 h in KRB buffer (1 mmol/l glucose) supplemented with either 1 μmol/l PMA, 400 μmol/l diazoxide or 2 μmol/l isradipine (all from Sigma-Aldrich, Stockholm, Sweden). After incubation, the cells were fixed with 4% (wt/vol.) paraformaldehyde in Ca2+-free PBS for 1 h and permeabilised with 5% (vol./vol.) Triton X-100 overnight. Unspecific binding was blocked with 5% (vol./vol.) donkey serum (Jackson Immunoresearch Laboratories, Avondale, PA, USA) before incubating with different primary antibodies overnight. The primary antibodies were rabbit anti-PKCα (1:250; Abcam, Cambridge, UK), rabbit anti-PKCδ (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, US), rabbit anti-PKCε (1:250; Abcam, Cambridge, MA, USA) and guinea pig anti-glucagon (1:500; Jackson ImmunoResearch Laboratories). After washing with PBS, the cells were exposed for 2 h to the following secondary antibodies: DyLight 488 AffiniPure Donkey Anti-rabbit IgG (1:100) and anti-guinea pig Cy 5 (1:50; both from Jackson ImmunoResearch Laboratories). The labelled cells were viewed using a confocal microscope (LSM510; Zeiss, Jena, Germany) using the 488 nm (Cy2) and 633 nm (Cy 5) lasers for excitation. Unspecific binding of the secondary antibodies was excluded by control experiments performed in the absence of the primary antibodies. The localisation of PKCα and PKCδ was quantified by the ratio between the mean fluorescent intensity in an area within 0.5 µm from the plasma membrane (I 1) and the cytosolic region (I 2), where \( {I_1} = \left( {s1 - s2} \right)/\left( {a1 - a2} \right) \) and \( {I_2} = \left( {s2 - s3} \right)/\left( {a2 - a3} \right) \). Here s1 is the total fluorescence intensity in the cell, s2 is fluorescence in the cytosol and nuclear area, and s3 is the fluorescence in the nuclear area; a1 is the total area of the cell, a2 is the area of cytosol and nuclear section, and a3 is the area of nucleus.
Data analysis
The increase in cell membrane capacitance was measured once a steady-state level had been attained. All data are quoted as mean values ± SEM of the indicated number of experiments (n). Statistical significances were evaluated using Student’s t test or two-way ANOVA.
Results
The effect of PKC-activation and inhibition on glucagon release
We measured glucagon secretion from intact mouse islets (Fig. 1a) at 1 or 8.3 mmol/l glucose with or without PMA (1 µmol/l), an artificial analogue of diacylglycerol and/or the PKC-antagonist, BIM (2.4 µmol/l). In agreement with previous studies [14, 35], islets incubated at 8.3 mmol/l glucose secreted less glucagon than those exposed to 1 mmol/l glucose (p < 0.01; n = 6). Application of PMA to islets exposed to 1 mmol/l glucose enhanced glucagon secretion about fivefold (p < 0.01; n = 6). PMA also stimulated glucagon secretion in the presence of 8.3 mmol/l glucose, albeit to a lesser extent than at 1 mmol/l glucose. The stimulatory action of PMA was abolished in the presence of BIM, regardless of whether the islets were incubated at 1 or 8.3 mmol/l glucose. BIM alone reduced glucagon secretion from mouse islets elicited by low glucose (1 mmol/l) by about 60% (p < 0.05, n = 6). Similar results were obtained in human islets (Fig. 1b) with the exception that BIM alone did not reduce glucagon secretion at 1 mmol/l glucose. Collectively, these data implicate PKC in the modulation of glucagon secretion from mouse and human alpha cells.
PKC-dependent glucagon secretion. (a) Glucagon release from mouse islets and (b) human islets was measured at 1 and 8.3 mmol/l glucose in the presence and absence of PMA (1 µmol/l) and/or BIM (2.4 µmol/l) as indicated. Data are the mean ± SEM of six experiments with mouse islets and 18 to 25 experiments with human islets (from six donors). *p < 0.05, **p < 0.01, ***p < 0.001 vs 1 mmol/l glucose or as indicated; †† p < 0.01, ††† p < 0.001 vs 8.3 mmol/l glucose or as indicated
Treatment with PMA stimulates pancreatic alpha cell exocytosis
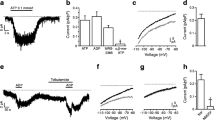
High-resolution capacitance perforated patch measurements were applied to single mouse alpha cells to investigate the effect of PKC activation on exocytosis elicited by trains of five 500-ms depolarisations from −70 to 0 mV (Fig. 2). PMA (10 nmol/l) augmented the exocytotic responses regardless of the magnitude of the responses under basal condition (Fig. 2a, b). The total capacitance increase evoked by the trains averaged 40 ± 15 fF (n = 12) under control condition and 185 ± 25 fF (p < 0.001) (Fig. 2c) 6 min after addition of PMA. Exocytosis elicited by this stimulation paradigm reflects fusion of granules belonging to the readily releasable pool (RRP) and its replenishment from a reserve pool [13]. An upper estimate of RRP can be obtained from the sum of the capacitance increase elicited by the first two depolarisations of the train [36], whereas the response to the following three pulses was used to estimate exocytosis due to replenishment of RRP. The RRP was thus estimated to be 48 ± 30 fF (n = 12) and 113 ± 26 fF (p < 0.001) in the absence and presence of PMA, respectively. This was paralleled by a sixfold increase in membrane capacitance from 12 ± 6 fF (n = 12) to 75 ± 14 fF (p < 0.001) in the response reflecting refilling of RRP.
PMA-dependent stimulation of exocytosis in single mouse alpha cells. a Capacitance increase elicited by a train of five 500 ms depolarisations from −70 to 0 mV before (black trace) and 6 min after (grey trace) inclusion of 10 nmol/l PMA in the extracellular solution. b Capacitance increase determined as above (a) for an alpha cell with a larger response in the absence of PMA. c Histogram summarising the average increase in cell membrane capacitance (ΔC m) evoked by all five depolarisations of the train, the first two depolarisations (RRP) and the following three depolarisations (refilling from reserve pool) in the presence and absence of PMA. d Peak Ca2+ current amplitude (I) against membrane potential (V) recorded before (white symbols) and after the addition of 10 nmol/l PMA in the extracellular solution (black symbols). Data are the mean ± SEM of 12 paired experiments. ***p < 0.001 vs control
To rule out the possibility that PMA stimulates alpha cell exocytosis by increased Ca2+ channel activity, we measured whole cell Ca2+ currents in response to depolarisations from the holding membrane potential −70 mV to membrane potentials ranging from −60 to +50 mV. However, no effect of PMA on the peak current amplitude was observed (Fig. 2d).
Treatment with BIM suppresses pancreatic alpha cell exocytosis
We next compared exocytosis in mouse alpha cells in the absence and presence of the PKC antagonist, BIM. In the majority of cells investigated (six out of nine cells), BIM reduced exocytosis evoked by a train of depolarisations (Fig. 3a). In these six cells, the total capacitance increase evoked by the train decreased from 138 ± 15 fF under control condition to 59 ± 10 fF (n = 6; p < 0.05) (Fig. 3b) in the presence of BIM. Further analysis (see above) revealed that BIM reduced RRP by around 60% (p < 0.05) (Fig. 3b) and the capacitance response reflecting refilling by around 40% (p < 0.05) (Fig. 3b). BIM did not affect exocytosis in the remaining three cells, in which exocytosis had already been low under control conditions (Fig. 3c).
Inhibition of exocytosis and voltage-dependent Ca2+ currents by BIM. a Increase in membrane capacitance of a mouse alpha cell with a high exocytotic response under control conditions (black trace) and 6 min after (grey trace) inclusion of 2.4 μmol/l BIM in the extracellular solution. Experiments were performed as described in the legend to Fig. 2. b Histogram summarising the average increase in cell membrane capacitance (ΔC m) measured during the experiment described in part a. c Capacitance increase in a mouse alpha cell where BIM had no further effect. d Peak Ca2+ current amplitude (I) against membrane potential (V) recorded before (black symbols) and after addition of 2.4 μmol/l BIM to the extracellular solution (white symbols). Data are mean ± SEM of six to eight paired experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs control
We also measured whole cell Ca2+ currents in the presence and absence of BIM (Fig. 3d). In eight cells, the Ca2+ current was inhibited by about 30% (p < 0.01).
Expression of genes encoding PKC isoforms in mouse and human islets
We investigated the gene expression pattern of different PKC isoforms in mouse and human pancreatic islets using quantitative real-time PCR (Fig. 4). Genes encoding classical PKC isoforms (PKCα, PKCβ1) were the most highly expressed in mouse, whereas those encoding the novel (PKCδ, PKCε, PKCη, PKCθ) and atypical PKC (PKCι, PKCζ) isoforms were more abundant in human islets.
Due to the extremely low expression of PKCβ1(also known as PRKCB or PRKCB1) in human islets, we selected PKCα for further analysis of activation and translocation of classical PKC isoforms in alpha cells. Among the novel PKC isoforms, PKCδ and PKCε were highly abundant in both species and have been described as being involved in exocytosis [28, 37]. We opted for PKCδ in our further study, since PKCε staining showed very low levels by confocal immunocytochemistry in mouse alpha cells (data not shown).
PMA- and Ca2+-dependent translocation of PKCα in mouse and human alpha cells
The distribution of PKCα was analysed by confocal microscopy on single mouse and human alpha cells exposed to 1 mmol/l glucose (Figs 5 and 6), i.e. conditions associated with a high rate of glucagon secretion (Fig. 1). The identity of the alpha cells was established by immunoreactivity for glucagon. The translocation of PKC isoforms was quantified by calculating the ratio of the fluorescent intensity in the vicinity of the plasma membrane (within a distance of 0.5 μm to the plasma membrane, I 1) to that in the inner cytosolic area (I 2) as illustrated in Fig. 5c (details, see Methods).
Distribution of PKCα in mouse alpha cells. a Confocal immunostaining of single alpha cells subjected to incubation in the absence (control) or presence of 1 µmol/l PMA as indicated. Cells were labelled with antibodies against PKCα (green) and glucagon (purple), and individual images of these merged. Pancreatic alpha cells were identified by glucagon counterstaining. b Treatment as in part a, except that cells were subjected to incubation with 400 μmol/l diazoxide, 2 μmol/l isradipine or isradipine in combination with 1 μmol/l PMA as indicated. Scale bars 2 μm; representative images of 10 to 15 scanned fields are presented. c Illustration of the areas used to calculate PKCα distribution ratio (I 1/I 2, see Methods). d Quantification of the distribution of PKCα in mouse alpha cells. Ctrl, control; Dia, diazoxide; Isr, isradipine. *p < 0.05 vs in presence of diazoxide. ***p < 0.001 vs control
Distribution of PKCα in human alpha cells. a, b Experiments as in Fig. 5a, b, but performed on human samples. Scale bars 2 μm, representative images of 10 to 15 scanned fields are presented. c Quantification of the distribution of PKCα in human alpha cells. Ctrl, control; Dia, diazoxide; Isr, isradipine. ***p < 0.001 vs control
In the absence of PMA, PKCα was located in the vicinity of the plasma membrane in about 90% of the mouse alpha cells (Fig. 5a, d), while only a few cells displayed cytosolic localisation (data not shown). This pattern did not change following stimulation with PMA (Fig. 5a, d).
Previous studies have shown that PKCα is Ca2+-sensitive [38]. To determine whether the association of PKCα with the plasma membrane observed in the presence of 1 mmol/l glucose alone is Ca2+-dependent, we treated mouse pancreatic cells with 400 μmol/l of the KATP-channel opener diazoxide or 2 μmol/l of the L-type Ca2+ channel inhibitor, isradipine, in the absence and presence of 1 μmol/l PMA. Confocal microscopy showed that exposure to diazoxide caused a re-distribution of PKCα from the cell membrane to the cytosol and decreased the ratio (I 1/I 2) by 50% (p < 0.001) (Fig. 5b, d). In the presence of isradipine, the reduction of the ratio was 45% compared with control (p < 0.001) (Fig. 5b, d). Thus, the re-distribution to the cytosol by diazoxide can almost entirely be attributed to inhibition of Ca2+ entry via L-type Ca2+ channels. The isradipine-induced redistribution of PKCα was counteracted when the cells were simultaneously exposed to PMA (Fig. 5b, d). The latter experiment demonstrates that the translocation of PKCα to the plasma membrane can be triggered independently, either by increased Ca2+ influx through L-type Ca2+ channels or by activation of PKC by PMA.
In contrast to the situation in mouse, PKCα in human alpha cells principally located to the cytosol in the absence of PMA (Fig. 6a, c). PMA treatment translocated PKCα from the cytosol towards the cell periphery and the I 1/I 2 ratio increased around twofold (p < 0.001). The distribution of PKCα in human alpha cells was not affected by diazoxide or isradipine (Fig. 6b, c), and PMA remained capable of triggering the translocation of PKCα in the presence of isradipine (Fig. 6b, c).
Translocation and distribution of PKCδ in mouse and human alpha cells
The distribution of PKCδ in the presence of 1 mmol/l glucose alone was cytosolic in mouse (Fig. 7) and human alpha cells (Fig. 8). PMA stimulated translocation of PKCδ from the cytosol towards the plasma membrane, and I 1/I 2 ratio increased 1.5-fold in mouse (p < 0.01) (Fig. 7a, c) and twofold in human alpha cells (p < 0.001) (Fig. 7a, c).
Distribution of PKCδ in mouse alpha cells. a Confocal immunostaining of cells dispersed from single islets from mouse as in Fig. 5a, except that cells were labelled with antibodies against PKCδ (green) as indicated. b Treatment as in part a, but performed in the presence of 400 μmol/l diazoxide, 2 μmol/l isradipine or isradipine in combination with 1 μmol/l PMA as shown. Scale bars 2 μm; representative images of 10 to 15 scanned fields are presented. c Quantification of the distribution of PKCδ in mouse alpha cells. Ctrl, control; Dia, diazoxide; Isr, isradipine. ***p < 0.001 vs control
Distribution of PKCδ in human alpha cells. a, b As in Fig 7a, b, except that experiments were performed on human alpha cells. Scale bars 2 μm, representative images of 10 to 15 scanned fields are presented. c Quantification of the distribution of PKCδ in human alpha cells. Ctrl, control; Dia, diazoxide; Isr, isradipine. ***p < 0.001 vs control
Although PKCδ, unlike the conventional PKCs, does not have a Ca2+-sensing C2 domain, we examined the impact of diazoxide and isradipine in the presence or absence of PMA stimulation on the distribution of this isoenzyme (Figs 7 and 8). Neither diazoxide nor isradipine changed the distribution of PKCδ in mouse (Fig. 7b, c) or human (Fig. 8b, c) alpha cells, suggesting that the translocation of PKCδ is Ca2+-independent. PMA stimulated PKCδ translocation towards the plasma membrane even in the presence of isradipine (Figs 7b, c, 8b, c).
Discussion
Glucagon is released by regulated exocytosis initiated by an increase in intracellular Ca2+ concentration [6, 13]. Protein phosphorylation by protein kinases such as PKC plays an important modulatory role in exocytosis in beta cells [39] and is known to augment exocytosis in a variety of diverse secretory cells [23–25, 39, 40]. Here we have investigated the role of PKC in alpha cell exocytosis and also the translocation of PKCα and PKCδ upon stimulation by the diacylglycerol analogue, PMA.
The PKC-activator, PMA, strongly stimulated glucagon secretion in mouse and human islets (Fig. 1). This is in accordance with a previous report on rat islets [15]. Capacitance measurements confirmed the glucagon release data and showed that PMA enhanced depolarisation-evoked exocytosis fivefold without affecting the magnitude of the Ca2+ current (Fig. 2a, d). This is reminiscent of our previous observations in insulin-secreting beta cells [39] and suggests that PKC stimulates glucagon secretion at a late stage exerted at the level of exocytosis itself. Analysis of the responses to the individual depolarisations indicates that PMA increased the size and refilling of RRP, and that BIM had the opposite effects. Thus, PKC activation exerts a dual role. This is in agreement with earlier studies on chromaffin cells [36, 41]. Munc-18 is one of the central proteins involved at several levels in the exocytotic process [30] and has been shown to be phosphorylated by PKC [32]. Indeed, all our data on alpha cells are consistent with a scenario in which PKC-dependent phosphorylation of munc-18 promotes fusion, priming and docking of granules.
BIM inhibits PKC activity by competitive binding to the ATP-binding site of the catalytic domain [42]. Our patch-clamp measurements revealed that BIM, in addition to inhibiting depolarisation-evoked exocytosis by around 60% (Fig. 3a), reduced the Ca2+ current by 30% (Fig. 3d). Thus, the reduced exocytotic response can be due to a combination of (1) direct inhibition of exocytosis as such and (2) reduction of Ca2+-induced exocytosis, which is secondary to inhibited Ca2+ influx. It remains to be determined whether the effect of BIM on the Ca2+ current is mediated by inactivation of PKC or is a direct inhibitory action of the compound on Ca2+ channel activity. Indeed, it has been demonstrated that BIM directly (i.e. not through inhibition of PKC activity) reduces voltage-dependent K+-channel currents in other cell types [43, 44]. Interestingly, the changes in the magnitude of the Ca2+ current were only obtained with BIM. In this context, we stress that PMA was without effect on the Ca2+ current (Fig. 2d). Thus, increased Ca2+ current is not necessary for PMA-stimulated exocytosis, and once PKC is activated by PMA, translocation not only of PKCδ, but also of PKCα can occur independently of Ca2+ influx (Figs 4b, 5b).
It has been shown that PKC increases its membrane affinity upon stimulation. Activation of PKC by diacylglycerol or PMA induces translocation of PKC from the cytoplasm to the plasma membrane [45]. Indeed, PKCδ translocated from cytosol to cell periphery upon PMA stimulation in mouse and human alpha cells (Figs 7 and 8). This re-distribution places PKCδ in close proximity to any downstream substrates involved in exocytosis. Our data indicate that translocation of PKCδ is Ca2+-independent, which is in agreement with a previous report that translocation of novel PKCs like PKCδ depends solely on binding of diacylglycerol to the C1 domains of PKCδ [46].
Interestingly, the translocation pattern of PKCα differs between mouse and human alpha cells. In mouse alpha cells, PKCα is preferentially located in the vicinity of the plasma membrane when exposed to glucose alone at concentrations as low as 1 mmol/l (Fig. 5a), while activation by PMA was necessary for the translocation of PKCα to the cell membrane in human alpha cells (Fig. 6a). This echoes the effect of BIM on glucagon secretion at 1 mmol/l glucose, which was only affected in mouse islets (Fig. 1b). It is tempting to speculate that this is a reflection of PKCα being tonically activated in mouse alpha cells and thereby susceptible to inhibition by BIM. PKCα, which belongs to the classical PKCs, is activated by increased intracellular Ca2+ and diacylglycerol [46]. In beta cells, glucose has been reported to produce a Ca2+-dependent translocation of classical PKCs to the plasma membrane [29]. In mouse alpha cells, we attribute the near-plasma membrane localisation of PKCα in the absence of PMA to the high [Ca2+]i resulting from Ca2+ influx through Ca2+ channels, which are activated during the action potentials generated at low glucose. This scenario is suggested by the redistribution of PKCα to the cytosolic region in cells exposed to diazoxide or isradipine (Fig. 5b). We demonstrated that Ca2+ influx through the L-type Ca2+ channel is the major source (90%) responsible for the Ca2+-dependent translocation of PKCα, while only 10% of the translocation is due to Ca2+ influx through other channels. We can only speculate about why this does not occur in human alpha cells, but it may be pertinent that distribution of PKCα is less peripheral in human than in mouse alpha cells (Figs 5 and 6). It is possible that in human alpha cells, the intracellular Ca2+ concentration is lower than in mouse alpha cells [47, 48], resulting in translocation of PKCα to the plasma membrane in a less Ca2+-dependent manner. This explanation would also account for the greater sensitivity to PMA.
Glucagon secretion and exocytosis in the pancreatic alpha cell triggered by low glucose alone is dependent on influx through the N-type Ca2+ channel [14]. Although L-type Ca2+ channels are not essential for exocytosis in alpha cells in the absence of high concentrations of cyclic AMP, the data presented here indicate that these channels mediate the Ca2+ influx that induces the Ca2+-dependent translocation of PKCα to the plasma membrane. Thus, L- and N-type Ca2+ channels fulfil different functions in the alpha cell (PKC translocation and exocytosis, respectively). We finally point out that elevation of intracellular cyclic AMP causes an interesting switch in Ca2+ channel dependence and that in the presence of forskolin or adrenaline, L-type Ca2+ channels account for most of the Ca2+ entry involved in exocytosis [6]. Further studies are required to elucidate the interaction between cyclic AMP/PKA- and PKC-mediated effects on exocytosis, and how they depend on Ca2+ influx and PKC translocation. Clearly, the control of alpha cell exocytosis is very complex, making it easy to envisage disturbances capable of causing defective regulation of glucagon secretion, which is a hallmark of type 2 diabetes [49].
Abbreviations
- BIM:
-
Bisindolylmaleimide
- Munc-18:
-
Mammalian homologue of Unc-18
- PKC:
-
Protein kinase C
- PMA:
-
Phorbol 12-myristate 13-acetate
- RRP:
-
Readily releasable pool
References
Lins PE, Wajngot A, Adamson U, Vranic M, Efendic S (1983) Minimal increases in glucagon levels enhance glucose production in man with partial hypoinsulinemia. Diabetes 32:633–636
Myers SR, Diamond MP, Adkins-Marshall BA, Williams PE, Stinsen R, Cherrington AD (1991) Effects of small changes in glucagon on glucose production during a euglycemic, hyperinsulinemic clamp. Metabolism 40:66–71
Cryer PE (2004) Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 350:2272–2279
Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA (2000) Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 85:4053–4059
Gromada J, Franklin I, Wollheim CB (2007) Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28:84–116
Gromada J, Bokvist K, Ding WG et al (1997) Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. J Gen Physiol 110:217–228
Vieira E, Liu YJ, Gylfe E (2004) Involvement of alpha1 and beta-adrenoceptors in adrenaline stimulation of the glucagon-secreting mouse alpha-cell. Naunyn Schmiedebergs Arch Pharmacol 369:179–183
Dunning BE, Foley JE, Ahren B (2005) Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia 48:1700–1713
Gromada J, Hoy M, Buschard K, Salehi A, Rorsman P (2001) Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G(i2)-dependent activation of calcineurin and depriming of secretory granules. J Physiol 535:519–532
Ma X, Zhang Y, Gromada J et al (2005) Glucagon stimulates exocytosis in mouse and rat pancreatic alpha-cells by binding to glucagon receptors. Mol Endocrinol 19:198–212
Gopel S, Zhang Q, Eliasson L et al (2004) Capacitance measurements of exocytosis in mouse pancreatic alpha-, beta- and delta-cells within intact islets of Langerhans. J Physiol 556:711–726
Rorsman P, Hellman B (1988) Voltage-activated currents in guinea pig pancreatic alpha 2 cells. Evidence for Ca2+-dependent action potentials. J Gen Physiol 91:223–242
Barg S, Galvanovskis J, Gopel SO, Rorsman P, Eliasson L (2000) Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes 49:1500–1510
MacDonald PE, De Marinis YZ, Ramracheya R et al (2007) A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol 5:e143
Hii CS, Stutchfield J, Howell SL (1986) Enhancement of glucagon secretion from isolated rat islets of Langerhans by phorbol 12-myristate 13-acetate. Biochem J 233:287–289
Bjaaland T, Hii CS, Jones PM, Howell SL (1988) Role of protein kinase C in arginine-induced glucagon secretion from isolated rat islets of Langerhans. J Mol Endocrinol 1:105–110
Shirai Y, Saito N (2002) Activation mechanisms of protein kinase C: maturation, catalytic activation, and targeting. J Biochem 132:663–668
Wolf BA, Easom RA, Hughes JH, McDaniel ML, Turk J (1989) Secretagogue-induced diacylglycerol accumulation in isolated pancreatic islets. Mass spectrometric characterization of the fatty acyl content indicates multiple mechanisms of generation. Biochemistry 28:4291–4301
Malaisse WJ, Dunlop ME, Mathias PC, Malaisse-Lagae F, Sener A (1985) Stimulation of protein kinase C and insulin release by 1-oleoyl-2-acetyl-glycerol. Eur J Biochem 149:23–27
Zawalich W, Brown C, Rasmussen H (1983) Insulin secretion: combined effects of phorbol ester and A23187. Biochem Biophys Res Commun 117:448–455
Stutchfield J, Jones PM, Howell SL (1986) The effects of polymyxin B, a protein kinase C inhibitor, on insulin secretion from intact and permeabilized islets of Langerhans. Biochem Biophys Res Commun 136:1001–1006
Easom RA, Hughes JH, Landt M, Wolf BA, Turk J, McDaniel ML (1989) Comparison of effects of phorbol esters and glucose on protein kinase C activation and insulin secretion in pancreatic islets. Biochem J 264:27–33
Hille B, Billiard J, Babcock DF, Nguyen T, Koh DS (1999) Stimulation of exocytosis without a calcium signal. J Physiol 520(Pt 1):23–31
Turner KM, Burgoyne RD, Morgan A (1999) Protein phosphorylation and the regulation of synaptic membrane traffic. Trends Neurosci 22:459–464
Leenders AG, Sheng ZH (2005) Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity. Pharmacol Ther 105:69–84
Hoy M, Berggren PO, Gromada J (2003) Involvement of protein kinase C-epsilon in inositol hexakisphosphate-induced exocytosis in mouse pancreatic beta-cells. J Biol Chem 278:35168–35171
Ganesan S, Calle R, Zawalich K et al (1992) Immunocytochemical localization of alpha-protein kinase C in rat pancreatic beta-cells during glucose-induced insulin secretion. J Cell Biol 119:313–324
Uchida T, Iwashita N, Ohara-Imaizumi M et al (2007) Protein kinase Cdelta plays a non-redundant role in insulin secretion in pancreatic beta cells. J Biol Chem 282:2707–2716
Yedovitzky M, Mochly-Rosen D, Johnson JA et al (1997) Translocation inhibitors define specificity of protein kinase C isoenzymes in pancreatic beta-cells. J Biol Chem 272:1417–1420
Toonen RF, Verhage M (2007) Munc18-1 in secretion: lonely Munc joins SNARE team and takes control. Trends Neurosci 30:564–572
Craig TJ, Evans GJ, Morgan A (2003) Physiological regulation of Munc18/nSec1 phosphorylation on serine-313. J Neurochem 86:1450–1457
Barclay JW, Craig TJ, Fisher RJ et al (2003) Phosphorylation of Munc18 by protein kinase C regulates the kinetics of exocytosis. J Biol Chem 278:10538–10545
Amisten S, Braun OO, Bengtsson A, Erlinge D (2008) Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb Res 122:47–57
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Salehi A, Vieira E, Gylfe E (2006) Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes 55:2318–2323
Gillis KD, Mossner R, Neher E (1996) Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron 16:1209–1220
Mendez CF, Leibiger IB, Leibiger B et al (2003) Rapid association of protein kinase C-epsilon with insulin granules is essential for insulin exocytosis. J Biol Chem 278:44753–44757
Nishizuka Y (1984) The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308:693–698
Ammala C, Eliasson L, Bokvist K et al (1994) Activation of protein kinases and inhibition of protein phosphatases play a central role in the regulation of exocytosis in mouse pancreatic beta cells. Proc Natl Acad Sci USA 91:4343–4347
Lindau M, Gomperts BD (1991) Techniques and concepts in exocytosis: focus on mast cells. Biochim Biophys Acta 1071:429–471
Nili U, de Wit H, Gulyas-Kovacs A et al (2006) Munc18-1 phosphorylation by protein kinase C potentiates vesicle pool replenishment in bovine chromaffin cells. Neuroscience 143:487–500
Toullec D, Pianetti P, Coste H et al (1991) The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266:15771–15781
Park WS, Son YK, Ko EA et al (2005) The protein kinase C inhibitor, bisindolylmaleimide (I), inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells. Life Sci 77:512–527
Kim A, Bae YM, Kim J et al (2004) Direct block by bisindolylmaleimide of the voltage-dependent K+ currents of rat mesenteric arterial smooth muscle. Eur J Pharmacol 483:117–126
Kraft AS, Anderson WB (1983) Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature 301:621–623
Mellor H, Parker PJ (1998) The extended protein kinase C superfamily. Biochem J 332(Pt 2):281–292
Quesada I, Todorova MG, Alonso-Magdalena P et al (2006) Glucose induces opposite intracellular Ca2+ concentration oscillatory patterns in identified alpha- and beta-cells within intact human islets of Langerhans. Diabetes 55:2463–2469
Nadal A, Quesada I, Soria B (1999) Homologous and heterologous asynchronicity between identified alpha-, beta- and delta-cells within intact islets of Langerhans in the mouse. J Physiol 517(Pt 1):85–93
Ali S, Drucker DJ (2009) Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. Am J Physiol Endocrinol Metab 296:E415–E421
Acknowledgements
We thank B.-M. Nilsson (Lund University Diabetes Centre) for expert technical assistance. This work was supported by the Swedish Research Council, EU-Biosim, the Knut and Alice Wallenberg foundation, the Crafoord foundation, the Thurings foundation, the Novo Nordisk Foundation and the Albert Påhlssons Foundation. P. Rorsman is supported by the Welcome Trust and the Medical Research Council. L. Eliasson and E. Renström are Swedish Research Council senior researchers.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Marinis, Y.Z., Zhang, E., Amisten, S. et al. Enhancement of glucagon secretion in mouse and human pancreatic alpha cells by protein kinase C (PKC) involves intracellular trafficking of PKCα and PKCδ. Diabetologia 53, 717–729 (2010). https://doi.org/10.1007/s00125-009-1635-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1635-x