Abstract
Aims/hypothesis
Intra-abdominal transplantation of non-visceral adipose tissue in rodents, simulating increased abdominal fat in obesity, paradoxically improves glucose tolerance and insulin sensitivity. We hypothesised that this improvement is due to transplant-induced enhanced uptake of fatty acids by adipose tissue, thus reducing fatty acid flux into, and triacylglycerol storage in, the liver.
Methods
In Experiment 1, mice were sham-operated or received heterologous epididymal white adipose tissue (WAT; EWAT) or visceral WAT (VWAT) transplantation to the portal and splanchnic circulation regions in the visceral cavity. In Experiment 2, inguinal WAT (IWAT) or EWAT was removed and subsequently transplanted to the visceral cavity of the same mouse (autotransplant). IWAT and EWAT autotransplants were repeated in Experiment 3 and compared with heterotransplants.
Results
Heterotransplantation of VWAT did not alter glucose tolerance, whereas auto- or hetero-transplantation of EWAT or IWAT significantly improved glucose tolerance. Transplantation-induced improvements in glucose tolerance 4 weeks after surgery coincided with decreased liver triacylglycerol, decreased portal plasma lipids and increased hepatic insulin sensitivity. By 8 weeks, these changes were apparent only in mice with autotransplantation. Heterologous EWAT transplantation-induced glucose improvement persisted without altered liver metabolism.
Conclusions/interpretation
Increases in visceral fat, via transplantation of visceral or non-visceral adipose tissue, is not a major risk factor for glucose intolerance. In fact, there are dynamic metabolic improvements following transplantation that include decreased portal lipids and improved liver metabolism, but these improvements are transient under certain circumstances.
Similar content being viewed by others
Introduction
Visceral obesity, the excessive accumulation of mesenteric and omental fat in humans, is presumed to predispose to adverse health consequences because of its venous drainage to the liver, i.e. insulin-sensitive hepatocytes are directly exposed to the metabolites and secretory products released by visceral adipocytes [1–3]. Because substrate delivery is a major determinant of both hepatic gluconeogenesis and VLDL synthesis [4], increased visceral fat, potentially releasing more NEFAs, glycerol, lactate and adipokines into the hepatic portal circulation, would be expected to have a major influence on these hepatic processes. Although the fundamental link between visceral fat and metabolic dysregulation is not known, increased intrahepatic triacylglycerol is a highly correlated indicator of obesity-related dysfunction [5].
Studies in rodents have demonstrated that implantation of different fat depots causes differential changes in glucose tolerance and insulin sensitivity [6, 7], implying that adipocytes from distinct anatomical locations are intrinsically different. Consistent with this, human visceral adipocytes have increased lipolysis and decreased sensitivity to the anti-lipolytic action of insulin, and thus a higher turnover of triacylglycerol, than subcutaneous adipocytes [8]; and lipoprotein lipase, fatty acid transporters and lipogenic genes are all significantly lower in epididymal than subcutaneous adipocytes in rodents [9].
Previous lipectomy and transplant studies in rodents are not easily translated to normal human physiology because of ambiguity as to which abdominal adipose depots are considered visceral, i.e. because epididymal white adipose tissue (EWAT) in male rodents is in an intra-abdominal location, EWAT is often considered to be a visceral depot. However, because it drains into systemic circulation and not the hepatic portal circulation, EWAT should not be considered a visceral depot [10]. In addition, EWAT has no human equivalent. Thus, it is more appropriate to conduct rodent lipectomy or transplant studies that manipulate adipose depots more comparable in location and function with those in humans. To test this, the aim of Experiment 1 was to identify intrinsic differences in hepatic metabolism and glucose tolerance after heterotransplantation of visceral WAT (VWAT) or EWAT.
An important question is whether the risk of metabolic complications related to excess visceral fat is due to its anatomical location or to intrinsic properties of the adipocytes themselves. It has been reported that transplanting subcutaneous inguinal WAT (IWAT) into the visceral cavity improved glucose tolerance, whereas transplanting EWAT into the same location did not [6, 7], although there are reports that EWAT heterotransplantation does improve glucose tolerance [11, 12]. These studies imply that the beneficial effects of adipose tissue transplantation can be due to both the intrinsic properties of the transplanted tissue as well as the destination within the body. Experiment 2 compared the metabolic benefits of IWAT or EWAT manipulation using the more sensitive procedure of autotransplantation, which enables assessment of physiological alterations that occur as the result of both fat removal and transplantation without altering total body fat. In Experiment 3, we further evaluated the effects of autologous and heterologous transplantation of IWAT or EWAT on hepatic insulin sensitivity and portal lipid concentrations.
Methods
Animals
Adult male C57BL/6 and enhanced green fluorescent protein (EGFP-C57BL/6) mice (Stock no. 005070; Jackson Laboratory, Bar Harbor, ME, USA; ~20 g) were individually housed under controlled conditions (12:12 light/dark cycle, 50–60% humidity and 25°C) with free access to low-fat pelleted chow (Harlan Teklad LM485, Madison, WI, USA) and water. Body mass was recorded before surgery and every 4 weeks after surgery. Procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Lipectomy, adipose tissue transplantation and sham surgeries
Mice were anaesthetised with isoflurane and injected subcutaneously with buprenex analgesic (300 μg/kg). Intra-abdominal fat removal and/or transplant surgeries were executed through a mid-ventral abdominal incision. For EWAT removal, testes were visualised and the attached fat pads were isolated and excised. VWAT was excised from the fundus and greater curvature of the stomach (portal VWAT) as well as from multiple locations along the mesentery proper (splanchnic VWAT). For IWAT removal, the skin of the dorsal hindlimb was separated from the musculature, and the fat was removed. All excised tissue was weighed. For sham surgery, a mid-ventral abdominal incision was made, organs were visualised and briefly exposed, and a minuscule drop of Vetbond tissue adhesive (3M Center, St Paul, MN, USA) was placed on the stomach and caecum before closure. Control animals (n = 8) were the same in Experiments 1 and 2. Care was taken not to disrupt major vasculature, musculature and, with EWAT removal, the testes.
In Experiment 1, ~0.10 g of donor VWAT (n = 8) or EWAT (n = 9) from transgenic EGFP-C57BL/6 mice was transplanted into the visceral cavity. In Experiment 2, ~0.15 g of EWAT (n = 5) or IWAT (n = 5) was removed and autotransplanted. In Experiment 3, ~0.15 g of tissue was removed for autologous EWAT (n = 10) and IWAT (n = 10) transplantation, and for heterologous EWAT (n = 10) and IWAT (n = 10) transplantation. Intra-abdominal transplants were attached via Vetbond to visceral fat attached at a location just anterior to the lesser gastric curvature, thus in close proximity to the hepatic artery and to visceral fat that is attached to the caecum in close proximity to the splanchnic circulation. Muscle and skin were closed with absorbable suture.
Glucose tolerance test
Intraperitoneal glucose tolerance tests (GTTs) were conducted before surgery and every 4 weeks after surgery. After an overnight fast (16 h), baseline blood glucose was obtained from the tail vein and 250 μl blood was collected. Mice then received a 1.5 mg/kg dextrose injection. Blood glucose was assessed 15, 30, 45, 60 and 120 min after injection.
Body composition analysis
Body composition was analysed with a quantitative nuclear magnetic resonance analyser (Echo MRI Whole Body Composition Analyzer; Echo Medical Systems, Houston, TX, USA) at the end of all the experiments.
EGFP adipose tissue: activation and visualisation
The transgenic EGFP-C57BL/6 mice used for donor tissue in Experiment 1 had an inducible Fas suicide/apoptotic system driven by the mouse colony-stimulating factor 1 receptor (Csf 1 r) promoter. This transgene contains mutations in the gene encoding binding protein (FKBP 12 ) in humans, which preferentially binds to the dimerisation drug AP20187, thus inducing apoptosis in macrophages and permitting fluorescence. At 3 h before tissue collection 9 weeks after surgery, transgenic mice were injected i.p. with AP20187 (5 mg/kg; ARIAD Pharmaceuticals, Cambridge, MA, USA) to permit fluorescence and consequently enable microscopic visualisation of EGFP under ultraviolet illumination. This was used to verify viability via revascularisation in the recipient.
Termination procedures (blood collection and tissue harvesting)
For Experiments 1 and 2, mice fed ad-lib were decapitated after an injection of Fatal Plus (Vortech Pharmaceutical, Dearborn, MI, USA) 9 weeks after surgery. Trunk blood was collected and plasma was stored at −80°C. One piece of liver was placed into RNAlater solution (Ambion, Austin, TX, USA) and another was snap-frozen and stored at −80°C. IWAT, EWAT, retroperitoneal WAT (RWAT) and VWAT were dissected and weighed (bilateral depots were summed) and stored in 4% paraformaldehyde.
Quantitative lipid assays
Commercially available kits were used to measure plasma triacylglycerol (Randox Laboratories, Crumlin, UK), cholesterol (Thermo Fisher Scientific, Middletown, VA, USA) and NEFAs (Wako, Richmond, VA, USA) per the manufacturers’ instructions.
Adipose tissue histology
WAT histology was performed according to the method of Foster and Bartness [13]. Each pad was sliced across its extent at 10 μm using a rotary microtome (American Optical Instrument, Buffalo, NY, USA). Slides were grouped into levels of approximately <100 μm, and five slides with three slice sections from each sample were analysed by light microscopy for average cell size using ImageJ (NIH, Bethesda, MD, USA).
Quantitative RT-PCR
RNA isolation and cDNA synthesis
During liver collection, the caudate lobe was immediately immersed in RNALater (1.5 ml) and held at −80°C as previously described [14]. RNAeasy columns (Qiagen, Valencia, CA, USA) were used to isolate RNA according to the manufacturer’s instructions. iScript (Bio-Rad, Hercules, CA, USA) was used to synthesise cDNA from 1 μg total RNA.
Quantitative real-time PCR
Primers for Lpl, Cpt-1a (also known as Cpt1a) or Mcp-1 (also known as Ccl2) were optimised as previously described [15] (table of primers: electronic supplementary material [ESM] Table 1). Samples were run in triplicate using an iCycler (Bio-Rad) and the iQ SYBR Green Supermix (Bio-Rad). Expression patterns of genes of interest were normalised to constitutively produced ribosomal protein L32, and relative expression was quantified as previously described [15].
Western blot (hepatic insulin sensitivity)
At 4 weeks after surgery in Experiment 3, 4 h fasted mice were administered i.p. saline or insulin (1 mU/g; SAFC Biosciences, Lenexa, KS, USA). After 15 min, the mice were anaesthetised with isoflurane and ~150 μl blood was collected from the hepatic portal vein. The mice were then killed, and a portion of the right lobe of the liver was snap-frozen in liquid nitrogen and stored at −80°C. Protein was subsequently extracted via homogenisation, and total AKT (Primary-1:1000; Cell Signaling, Danvers, MA, USA) and phospho-thymoma vital proto-ongogene (pAKT; 1:1000; Cell Signaling) protein production was assessed by western blot analysis via chemiluminescence-induced (GE Healthcare, Chalfont St Giles, UK) densitometry readings visualised using film. Density was determined using ImageJ. Background was subtracted from each sample, and pAKT samples were then normalised to total AKT and expressed as the percentage of respective saline-injected control.
Statistical analysis
Data are expressed as means±SEM. Comparisons among multiple groups on most dependent variables including AUC, lipid profile data, RT-PCR data (Lpl, Cpt-1a [also known as Cpt1a] or Mcp-1 [also known as Ccl2]), pAKT protein production and individual and total adipose mass were performed using one-way between-subjects ANOVA (SPSS for Windows, release 11.5.0; SPSS, Chicago, IL, USA). Intraperitoneal GTT data, with a group × time (3 × 6) design, were analysed using two-way ANOVA. Post hoc tests of individual groups were made using Tukey’s tests. For all experiments, differences among groups were considered statistically significant if p < 0.05. Exact probabilities and test values were omitted for simplicity and clarity of presentation of the results.
Results
Experiment 1: are metabolic complications related to excess visceral fat, such as glucose intolerance, due to the intrinsic properties of the visceral adipocytes themselves?
Glucose tolerance test
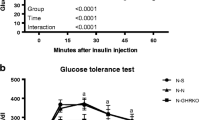
All groups were comparable before surgery (Fig. 1a) as well as 4 weeks after surgery (Fig. 1b). At 8 weeks, EWAT heterotransplantation resulted in a significantly decreased rise in blood glucose at 30, 45 and 60 min compared with controls (p ≤ 0.05, Fig. 1c), and at 60 min compared with mice receiving visceral heterotransplantation (p ≤ 0.05). The persistent diminution of glucose levels in the EWAT transplant group at 8 weeks resulted in a significant decrease in AUC (p ≤ 0.05; ESM Fig. 1c).
Experiment 1: glucose measurements (mmol/l) at 0, 15, 30, 45, 60 and 120 min after glucose injection (i.p.) before surgery (a) and 4 (b) and 8 (c) weeks after surgery. At 8 weeks after EWAT transplantation, blood glucose was decreased at 30, 45 and 60 min after glucose injection (*p ≤ 0.05 vs control and † p ≤ 0.05 vs control and VWAT transplantation). Diamonds, control; squares, VWAT heterotransplantation; triangles, EWAT heterotransplantation
Adipose and liver variables
Terminal mass of transplanted fat was not determined, although the bulk of the transplanted tissue was readily identifiable, i.e. it was attached to pre-existing adipose tissue making it difficult to accurately isolate along the junction. Therefore the mass of VWAT recovered in Table 1 is the sum of endogenous VWAT mass plus the transplanted tissue. VWAT heterotransplantation caused a significant increase in RWAT mass (p ≤ 0.05 vs control). VWAT and EWAT heterotransplantation caused a significant increase in VWAT mass (p ≤ 0.05). NMR fat and lean mass was not different among groups. VWAT, but not EWAT, heterotransplantation caused a significant increase in liver triacylglycerol deposition (p ≤ 0.05; Fig. 2). VWAT heterotransplantation, however, did not alter liver expression of Lpl, Cpt-1a or Mcp-1 mRNA (ESM Fig. 2). EWAT heterotransplantation resulted in a trend for an increase in liver Lpl expression (p = 0.07; ESM Fig. 2a), but no changes in Cpt-1a or Mcp-1 (ESM Fig. 2b,c).
Lipid profile and adipose tissue histology
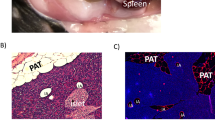
Heterotransplantation of fat from a donor mouse did not alter systemic plasma NEFA, triacylglycerol or cholesterol in the fed or fasted state (ESM Table 2). Transplants are considered successful if revascularised with a normal appearance both macro- and microscopically. Blunt dissection and close observation confirmed that the transplanted adipose tissue in both groups was viable and revascularised (Fig. 3a–c), and this was microscopically confirmed by the presence of green fluorescent protein (GFP). Figure 3d, g and i depict EWAT, portal VWAT and splanchnic VWAT tissue from control GFP-producing mice; under control conditions EWAT has the most robust GFP production, with splanchnic VWAT having a moderate amount and portal VWAT little to none. This same pattern occurred with transplanted tissue, with EWAT transplanted to the adipose tissue surrounding the caecum (Fig. 3e) and stomach (Fig. 3f) region of a recipient robustly producing GFP, with moderate production in transplanted splanchnic VWAT (Fig. 3h), and with little production in portal VWAT (Fig. 3j). Transplanted splanchnic VWAT, portal VWAT and EWAT had normal-appearing unilocular rings of fat cells, with intermittent macrophages and vascular cells (Fig. 4a–c). The mean relative areas (pixels) of transplanted adipocytes were the same as occurred in the respective control adipose tissue, although splanchnic VWAT and portal VWAT areas were significantly smaller than EWAT areas (p ≤ 0.05; Fig. 4d).
Experiment 1: adipose tissue histology and GFP verification of revascularisation. Transplanted tissue at blunt dissection was adhered to fat surrounding the stomach (a), caecum (b) and sometimes connected between the two sites (c). d, g and i depict control EWAT, splanchnic VWAT and portal VWAT GFP production. e GFP production in EWAT transplanted to the adipose tissue connected to the caecum and f lesser gastric curvature. h GFP production in splanchnic VWAT transplanted to adipose tissue connected to the caecum and j portal VWAT transplantation to adipose tissue connected to the lesser gastric curvature. Arrows indicate revascularisation of transplants
Experiment 1: adipose tissue histology—(a) EWAT, (b) splanchnic VWAT and (c) portal VWAT (×20 magnification image of haematoxylin-stained control adipose tissue). d Experiment 1: splanchnic and portal VWAT mean adipocyte area was significantly smaller than EWAT (different letters indicate significant difference, p ≤ 0.05). e Experiment 2: IWAT autotransplants, but not EWAT, had smaller adipocytes than respective control (*p ≤ 0.05 vs control). C, control; T, transplant
Experiment 2: are the beneficial metabolic effects of transplantation further enhanced with the combined removal of adipose tissue and autologous transplantation, and are effects depot-specific?
Glucose tolerance test
Pre-surgical GTTs were comparable among groups (Fig. 5a). At 4 and 8 weeks after surgery, both autotransplantation groups had decreased blood glucose compared with controls (p ≤ 0.05; Fig. 5b,c). More specifically, at 4 weeks after surgery, IWAT autotransplantation into the visceral region resulted in significantly decreased glucose from 15 to 120 min compared with both control and EWAT autotransplantation groups (p ≤ 0.05; Fig. 5b). EWAT autotransplantation caused a significant decrease in glucose at 30 and 45 min compared with controls. The persistent diminution of blood glucose in the IWAT group resulted in a significant group × time interaction (p ≤ 0.05), with the IWAT autotransplantation group having a significantly lower 4-week AUC (p ≤ 0.05; ESM Fig. 3b) compared with controls and a trend toward a significant decrease compared with EWAT autotransplantation.
Experiment 2: glucose measurements (mmol/l) at 0, 15, 30, 45, 60 and 120 min after glucose injection (i.p.) before surgery (a) and 4 (b) and 8 (c) weeks after surgery. b 4 weeks: IWAT autotransplantation significantly decreased blood glucose from 15 to 120 min. EWAT autotransplantation decreased blood glucose at 30 and 45 min. c 8 weeks: IWAT autotransplantation decreased glucose levels at 30, 45 and 60 min. EWAT autotransplantation decreases glucose at 45 and 60 min. † p ≤ 0.05 vs control and EWAT autotransplantation. *p ≤ 0.05 vs control. Diamonds, control; squares, EWAT autotransplantation; triangles, IWAT autotransplantation
At 8 weeks, IWAT autotransplantation mice had significantly decreased blood glucose at 30, 45 and 60 min (p ≤ 0.05; Fig. 5c). Glucose levels in EWAT mice were significantly lower than control levels at 45 and 60 min (p ≤ 0.05). The persistent reduction in blood glucose in the IWAT autotransplantation group resulted in a significant group × time interaction (p ≤ 0.05), with IWAT autotransplantation causing a significant decrease in 8-week AUC compared with controls (ESM Fig. 3c). EWAT autotransplantation also significantly decreased 8-week AUC compared with controls (ESM Fig. 3c).
Adipose and liver variables
IWAT mass in the IWAT autotransplantation group and EWAT mass in the EWAT autotransplantation group were significantly decreased compared with sham controls, as expected. IWAT autotransplantation significantly decreased VWAT mass compared with controls and the EWAT autotransplantation group (Table 1). IWAT autotransplantation significantly decreased total adipose tissue mass and NMR measured fat and lean mass compared with controls and EWAT autotransplantation (p ≤ 0.05; Table 1). Both autotransplantion groups had significantly reduced liver triacylglycerols, Lpl and Cpt-1a compared with controls (p ≤ 0.05; Fig. 6a–c, respectively). Liver Mcp-1 was significantly decreased in the EWAT autotransplantation group compared with controls (p ≤ 0.05; Fig. 6d), and approached a significant increase in the IWAT autotransplantation group (p = 0.06).
Experiment 2: autotransplantation of EWAT or IWAT significantly decreased liver triacylglycerol concentration (mmol/l) (*p ≤ 0.05 vs control) (a) and mRNA liver expression of Lpl (b) and Cpt-1a (c) as percentage of control. Mcp-1 was significantly decreased in the EWAT autotransplantation, but approached a significant increase (p = 0.06) in the IWAT group (*p ≤ 0.05 vs control) (d). Auto, autotransplantation
Lipid profile and adipose tissue histology
Plasma triacylglycerol concentration was significantly decreased in the IWAT autotransplantation group under fed, but not fasted, conditions compared with controls (p ≤ 0.05; ESM Table 2). Transplanted tissue was revascularised and had normal appearance. Autologous IWAT transplants, but not EWAT transplants, had significantly decreased average adipocyte cell size (p ≤ 0.05; Fig. 4e).
Experiment 3: are transplantation-induced improvements in glucose tolerance due to alterations in hepatic insulin sensitivity?
All transplantation groups had significantly improved glucose tolerance (p ≤ 0.05; Fig. 7a) and significantly decreased liver triacylglycerol (p ≤ 0.05; Fig. 7b) 4 weeks after surgery. In addition, insulin-induced pAKT production was significantly increased in all transplanted groups compared with sham controls (p ≤ 0.05; Fig. 7c). Mice with IWAT autotransplantation had significantly increased pAKT production compared with those that received hetero- or auto-transplantation of EWAT (p ≤ 0.05). pAKT production of IWAT heterotransplantation was also significantly increased compared with the respective heterologous EWAT group (p ≤ 0.05) (AKT western blot image: ESM Fig. 4).
At 4 weeks after surgery, there was no difference in body, fat or lean mass (ESM Table 3). As expected, the mass of the respective relocated adipose depot was significantly reduced in the autologous groups compared with heterologous and sham control animals (p ≤ 0.05). Consistent with this, total dissected adipose tissue mass was significantly less in the autologous than in the heterologous groups (p ≤ 0.05). Although transplanted tissue was revascularised and normal in appearance, VWAT mass 4 weeks after transplantation was not increased.
Because systemic plasma lipids remained unchanged in Experiments 1 and 2, we predicted that intraportal sampling may be more informative. Analogous to systemic blood, mice that were saline-injected after a 4 h fast had normal portal lipids after transplantation (ESM Fig. 5a–c, saline injected). Conversely, animals with transplants that received insulin had significantly lower portal plasma lipids than sham controls (group main effect for triacylglycerol, cholesterol and NEFA; p ≤ 0.05; ESM Fig. 5a–c, insulin injected). More specifically, triacylglycerol was significantly reduced in all transplant groups, cholesterol was significantly decreased in the EWAT and IWAT heterologous and autologous EWAT groups, and autologous EWAT and heterologous IWAT transplantation significantly decreased NEFAs (p ≤ 0.05).
Discussion
All transplantations were made into the viscera where they would drain into the hepatic portal vein, and indices of glycaemia and hepatic metabolic function were determined. Our results show that heterologous VWAT transplantation into the visceral region did not alter glucose variables. In contrast, heterologous EWAT transplantation into the same region improved glucose tolerance. Because EWAT, however, has no human equivalent, we propose that it is more appropriate to manipulate adipose depots that are more comparable in location and function with those in humans and we also compared the relative effect of hetero- vs auto-transplantation. Autotransplantation of either IWAT or EWAT improved glucose tolerance, decreased liver triacylglycerol and reduced liver enzymes that are normally increased in obesity and/or fatty liver; thus IWAT transplantation is a suitable replacement for EWAT. Substantially earlier post-surgical termination revealed that glucose tolerance improvements following hetero- and auto-transplants of IWAT or EWAT coincided with decreased hepatic portal lipids (after insulin stimulation) and liver fat storage and increased liver insulin sensitivity. Overall, we have demonstrated a relationship between adipose transplant-induced improvements in glucose tolerance and reduced lipids into, and fat storage in, the liver.
Transplanting visceral adipose tissue from a lean donor into the visceral cavity of a lean recipient allows clarification of whether an increase in intra-abdominal fat per se is a risk factor for aspects of the metabolic syndrome. We found that incrementing visceral fat with more visceral fat does not affect glucose tolerance, whereas incrementing it with EWAT actually improves glucose tolerance. Thus, the inherent cellular characteristics of the two depots differ, and histological examination substantiated this (Fig. 4a–c) Hence, although transplanted EWAT is located intra-abdominally, it neither functions nor looks like visceral adipose tissue. It could be argued that relocating VWAT from one lean chow-fed mouse to another bears little relationship to what occurs in diet-induced abdominal obesity, i.e. on the low-fat diet, adipocytes are not challenged by the conditions of energy/fat excess and are thus are not prompted to hypertrophy. Indeed only liver triacylglycerol storage was increased following VWAT transplantation. Because increased adipocyte size is a predictor of insulin resistance [16], perhaps VWAT transplantation from a high-fat-diet-induced obese animal would impair glucose tolerance.
EWAT heterotransplantation into the abdominal cavity has been reported to improve glucose tolerance in some studies [11, 12], while others suggest no alterations [6, 7] or impaired glucose tolerance [17]. Inconsistencies may be due to numerous factors including amount of fat transplanted, duration of post-surgical recovery, revascularisation location and/or time of fasting. EWAT mass transplanted in prior studies varied from ~150 to 1,000 mg [6, 7, 11]. We used small (~100–150 mg) transplants because the occurrence of necrosis/transplant rejection increases with larger volumes (M.T. Foster, unpublished observation), and, in Experiment 1, we were limited to the amount of VWAT collected. Even with the small size, EWAT transplantation had improved glucose tolerance at 4 and 8 weeks, consistent with other observations after 10 weeks [11]. Proximity of the transplant to the portal vein also probably influences insulin sensitivity. If the transplant is anchored to the peritoneum, revascularisation drains into the systemic circulation [7, 11], whereas if attachment is directly to the visceral/mesenteric tissue, drainage is into the hepatic portal circulation as in the current study.
At 8 weeks after surgery, EWAT heterotransplanted mice continued to have improved glucose tolerance, but without alterations in liver metabolism. It is possible that EWAT-specific secretory factors, adipokines and sex steroids [18] independently influence glucose tolerance. Thus far, systemic plasma concentrations of adiponectin, leptin and resistin have been demonstrated to be unaltered 10 weeks after EWAT transplantation [11], but intraportal sampling may be more informative.
Therefore, although EWAT implantation does improve glucose tolerance, it may be more clinically applicable to manipulate adipose depots more homologous to humans. Autotransplantation, a common procedure for cosmetic and reconstructive surgery in humans, was used to compare the glucose-improving effects of IWAT and EWAT. Both improved glucose tolerance, but, unlike heterotransplantation of EWAT, there were differences in lipid metabolism. Both autotransplantation groups had decreased liver Lpl (the enzyme controlling NEFA entry into cells), Cpt-1a (the rate-limiting enzyme for mitochondrial β-oxidation of long-chain fatty acids) and liver triacylglycerol. Liver production of Mcp-, a marker of inflammation/macrophage infiltration, was the only hepatic measurement that was differentially regulated. Although there is no direct quantitative relationship between gene expression and protein synthesis, the increase in liver MC level may result from high IWAT Mcp-1 gene expression, which is about six times higher than that of EWAT and ~24 times higher than that of mesenteric WAT [19]. Another IWAT-specific confound is a post-surgical decrease in body and adipose mass and decreased adipocyte size, a finding consistent among IWAT heterologous transplant studies [6, 7]. Differential regulation among fat transplants may be attributable to site-related differences in adipokine release [19]; however, this was not tested.
To characterise opposing outcomes of auto- and hetero-transplantation, we compared IWAT and EWAT. At 4 weeks after surgery, all transplantations had improved glucose tolerance and improved liver lipid profile. Previous research indicates that IWAT-transplantation-induced glucose improvements coincide with enhanced insulin suppression of hepatic glucose production [6]; similarly, liver insulin sensitivity was increased in all transplantation groups in conjunction with decreases in liver triacylglycerol storage. Although portal plasma lipids did not differ among groups in the control condition, insulin decreased cholesterol, triacylglycerol and NEFA in the transplantation groups. Overall, improvements in insulin sensitivity following transplantation enhanced the ability of insulin to decrease cholesterol and triacylglycerol secretion from the liver [20–22] and decreased NEFA flux from adipose tissue [23]. At present, we do not know if transplant-induced improvements in lipid metabolism are a consequence of, or a contributing factor to, improved glucose tolerance.
Glycaemia-improving effects with or without altered liver function seem to occur independently of increased fatty acid storage in adipose tissue. Perhaps transplanted tissue does not increase the capacity of visceral fat storage, but does release factors that alter fat metabolism. These mechanisms need to be further investigated in a high-fat-diet-induced obese model.
Abbreviations
- AKT:
-
Thymoma vital proto-ongogene
- CPT-1A:
-
Carnitine palmitoyltransferase
- EGFP:
-
Enhanced green fluorescent protein
- EWAT:
-
Epididymal WAT
- GFP:
-
Green fluorescent protein
- GTT:
-
Glucose tolerance test
- IWAT:
-
Inguinal WAT
- LPL:
-
Lipoprotein lipase
- MCP-1:
-
Monocyte chemoattractant protein
- RWAT:
-
Retroperitoneal WAT
- WAT:
-
White adipose tissue
- VWAT:
-
Visceral WAT
References
Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM (1996) IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271:665–668
Bergman RN (2000) Non-esterified fatty acids and the liver: why is insulin secreted into the portal vein? Diabetologia 43:946–952
Williamson JR, Kreisberg RA, Felts PW (1966) Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A 56:247–254
Kissebah AH, Krakower GR (1994) Regional adiposity and morbidity. Physiol Rev 74:761–811
Fabbrini E, Magkos F, Mohammed BS et al (2009) Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A 106:15430–15435
Tran TT, Yamamoto Y, Gesta S, Kahn CR (2008) Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7:410–420
Hocking SL, Chisholm DJ, James DE (2008) Studies of regional adipose transplantation reveal a unique and beneficial interaction between subcutaneous adipose tissue and the intra-abdominal compartment. Diabetologia 51:900–902
Ostman J, Arner P, Engfeldt P, Kager L (1979) Regional differences in the control of lipolysis in human adipose tissue. Metabolism 28:1198–1205
del Mar RM, Fernandez-Lopez JA, Esteve M, Alemany M (2009) Site-related white adipose tissue lipid-handling response to oleoyl-estrone treatment in overweight male rats. Eur J Nutr 48:291–299
Harris RB, Leibel RL (2008) Location, location, location. Cell Metab 7:359–361
Konrad D, Rudich A, Schoenle EJ (2007) Improved glucose tolerance in mice receiving intraperitoneal transplantation of normal fat tissue. Diabetologia 50:833–839
Foster MT, Shi H, Seeley RJ, Woods SC (2010) Transplantation or removal of intra-abdominal adipose tissue prevents age-induced glucose insensitivity. Physiol Behav 101:282–288
Foster MT, Bartness TJ (2006) Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am J Physiol Regul Integr Comp Physiol 291:R1630–R1637
Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ (2008) Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab 294:E630–E639
Kim DH, Sandoval D, Reed JA et al (2008) The role of GM-CSF in adipose tissue inflammation. Am J Physiol Endocrinol Metab 295:E1038–E1046
Schneider BS, Faust IM, Hemmes R, Hirsch J (1981) Effects of altered adipose tissue morphology on plasma insulin levels in the rat. Am J Physiol 240:E358–E362
Rytka JM, Wueest S, Schoenle EJ, Konrad D (2011) The portal theory supported by venous drainage-selective fat transplantation. Diabetes 60:56–63
Khor VK, Tong MH, Qian Y, Song WC (2008) Gender-specific expression and mechanism of regulation of estrogen sulfotransferase in adipose tissues of the mouse. Endocrinology 149:5440–5448
del Mar RM, Fernandez-Lopez JA, Esteve M, Alemany M (2009) Different modulation by dietary restriction of adipokine expression in white adipose tissue sites in the rat. Cardiovasc Diabetol 8:42
Alexander CA, Hamilton RL, Havel RJ (1976) Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J Cell Biol 69:241–263
Penhos JC, Wu CH, Lemberg A et al (1968) The effect of insulin on the metabolism of lipids and on urea formation by the perfused rat liver. Metabolism 17:246–259
Alcindor LG, Infante R, Soler-Argilaga C, Polonovski J (1973) Effect of a single insulin administration on the hepatic release of triacylglycerol into the plasma. Biochim Biophys Acta 306:347–352
Holm C, Osterlund T, Laurell H, Contreras JA (2000) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr 20:365–393
Acknowledgements
This research was supported by National Institutes of Health: DK087816 (M.T. Foster), DK017844 (S.C. Woods), DK078201 (S.C. Woods) and DK54890 (R.J. Seeley).
Contribution statement
MTF designed the study, interpreted the data, drafted/revised the manuscript and approved final version of the manuscript. HS contributed to the concept and design, revisions and final approval of the manuscript. SS and RK contributed to interpretation of data, revisions and final approval of the manuscript. RJS and SCW contributed to conception and design, revisions and final approval of the published manuscript.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
Primer sequences (PDF 9 kb)
ESM Table 2
Experiments 1 and 2 fed triglyceride, NEFA and cholesterol concentrations and fasted triglyceride and NEFA in systemic plasma (PDF 41 kb)
ESM Table 3
Experiment 3: Body mass (g) and absolute dissected individual (mg) and total adipose tissue (mg) and NMR fat (mg) and lean mass (g), (PDF 47 kb)
ESM Figure 1
Experiment 1: Pre-surgery, 4 and 8 weeks post-surgery area under the curves. 8 weeks AUC was significantly lower in the EWAT hetero group compared with control (*P ≤ 0.05 vs control; PDF 54 kb)
ESM Figure 2
Experiment 1: Liver gene production of LPL (B.), CPT-1a (C.) and MCP-1 (D.) as percent of control (PDF 55 kb)
ESM Figure 3
Experiment 2: Pre-surgery, 4 and 8 week post-surgery area under the curves. (B.) 4 weeks: IWAT autotransplantation significantly decreased and AUC. (C.) 8 weeks: IWAT and EWAT autotransplantation decreased AUC. (*P ≤ 0.05 vs control; PDF 57 kb)
ESM Figure 4
Experiment 3: AKT western blot image verification (PDF 89 kb)
ESM Figure 5
Experiment 3: Lipid profile of portal plasma. Plasma portal lipids of saline-injected animals was normal following transplantation (Saline Injected A-C). Insulin-injected animals with transplants had significantly lower portal plasma lipids. Triglycerides were significantly reduced in all transplant groups (Insulin Injected A). Cholesterol was significantly decreased in the EWAT and IWAT heterologous and autologous EWAT groups (Insulin Injected B). Autologous EWAT and heterologous IWAT transplantation significantly decreased free fatty acids (Insulin Injected C). (Unlike letters indicates significance = P ≤ 0.05; PDF 72 kb)
Rights and permissions
About this article
Cite this article
Foster, M.T., Shi, H., Softic, S. et al. Transplantation of non-visceral fat to the visceral cavity improves glucose tolerance in mice: investigation of hepatic lipids and insulin sensitivity. Diabetologia 54, 2890–2899 (2011). https://doi.org/10.1007/s00125-011-2259-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2259-5