Abstract
Objective
To assess the potential of dexmedetomidine for targeted sedation in complex Intensive Care (ICU) patients for >24 h.
Design
Prospective, open label, clinical trial.
Setting
Tertiary general ICU.
Patients
Twenty critically ill patients, mean APACHE II 23(±9).
Interventions
A continuous infusion of dexmedetomidine, median infusion time 71.5 (35–168) h, starting at 0.4μg·kg·h without a loading dose and adjusted (0.2–0.7μg·kg·h) to a target Ramsay Sedation Score (RSS) of 2–4. Rescue midazolam and/or morphine/fentanyl were given as clinically indicated.
Measurements and results
Haemodynamic parameters and RSSs were collected until 24 h after cessation. An RSS 2–5 was achieved in 1,147 (83%) of observations with a reduction in RSS of 6 from 13% in the first 6 h to 3% between 18 h and 24 h. Sixteen patients needed minimal or no additional midazolam, median 4 mg/day (0.5–10) and ten required minimal or no additional analgesia, median 2 mg/day (0.5–4.5), 55μg/day (14–63) of morphine/fentanyl.
Results
A 16% reduction in mean systolic blood pressure (SBP) and 21% reduction in heart rate (HR) occurred over the first 4 h followed by minimal (± 10%) changes throughout the infusion. A rise in SBP was observed in two patients. After abrupt cessation, SBP and HR monitored for 24 h rose by 7% and 11%, respectively.
Conclusions
Dexmedetomidine was an effective sedative and analgesic sparing drug in critically ill patients when used without a loading dose for longer than 24 h with predictable falls in blood pressure and HR. There was no evidence of cardiovascular rebound 24 h after abrupt cessation of infusion.
Similar content being viewed by others
Introduction
Providing appropriate long-term sedation in mechanically ventilated critically ill patients is a challenging problem that faces many intensivists. Drugs that are used currently have known addictive and cumulative potentials. Although unquantified in the intensive care setting, the risk of dependence, emergence delirium, withdrawal phenomena, and drug accumulation is substantial. Current sedation practices have been associated with prolonged mechanical ventilation and increased ICU length of stay [1].
Dexmedetomidine, a highly selective α-2-receptor agonist, has been recently introduced for sedation in the ICU setting. It combines analgesic, sedative, and anxiolytic effects while maintaining patient rousability without significant respiratory depression [2]. The lack of addictive properties and withdrawal phenomena of α-2-receptor agonist makes longer-term sedation with dexmedetomidine attractive and may facilitate weaning from mechanical ventilation. There have been several studies describing its successful use in mechanically ventilated postoperative patients for short-term use (24 h) in the ICU setting [3, 4, 5, 6, 7]. More recently, dexmedetomidine use has been described in mechanically ventilated critically ill medical patients for up to 48 h [8]. It is approved in many countries for up to 24-h use in post-operative patients requiring mechanical ventilation.
To further investigate the potential role of dexmedetomidine as a longer-term sedative agent in intensive care, we studied the sedative and cardiovascular effects of a prolonged infusion of dexmedetomidine (up to 7 days) titrated to a target Ramsay Sedation Score (RSS), under conditions typical of our clinical practice, in 20 critically ill medical and surgical patients requiring mechanical ventilation.
Materials and methods
Patients
The South East Sydney Area Health Service Ethics Committee and the Australian Therapeutic Goods & Administration approved this study. Written informed consent was obtained for all patients from the person responsible for use longer than 24 h. The Ethics Committee did not require retrospective consent. Patients were enrolled if they met the following criteria: age over 18 years; mechanically ventilated, likely to require more than 24 h of intensive care with concomitant analgesia and/or sedation; and consent of the responsible physician. Exclusion criteria were: use of neuromuscular blocking agents during the study drug infusion period other than for the insertion of an endotracheal tube; known or suspected serious allergy to any medication that might be administered during the course of this study; current treatment with alpha-2-antagonist or agonist; participation in a trial with any other experimental drug within 30 days prior to admission to ICU; pregnancy or lactation; spinal or epidural catheter in use; or failure to obtain consent. Apart from the study drug, all other management was according to unit protocols.
Study drug
Dexmedetomidine HCl (Abbott Australasia) was supplied in a 2-ml ampoule containing 100μg/ml in sodium chloride solution. The drug was diluted with normal saline to a body mass corrected concentration, whereby 1 ml/h of infusion equal to 0.1μg·kg·h, and was administered through a syringe pump via a central venous line.
Measurements
Physical examination, ECG, and haematological and biochemical profiles were obtained on admission and daily whilst in the ICU. All concurrent medications were recorded including dose, frequency, and route of administration.
Haemodynamic (systolic and diastolic blood pressure, heart rate) measurements were obtained every 15 min in the first 2 h after commencement of dexmedetomidine and then hourly for the duration of the infusion. After cessation of the infusion, haemodynamic observations were continued for a further 24 h. A significant decrease in systolic blood pressure (SBP) was defined as a reduction of more than 30% from baseline (i.e., immediately prior to commencement of the infusion) and hypotension as a SBP of less than 90 mmHg. A significant fall in heart rate (HR) was defined as a reduction of more than 20% of baseline and severe bradycardia was defined as a HR of less than 50 beats per minute (bpm), regardless of the level of vasoactive drugs that the patient was receiving. Hypotension was treated with fluids initially and then with vasopressors. Sedation was assessed using the RSS.
Study protocol
Eligible patients received dexmedetomidine at an initial infusion rate of 0.4μg·kg·h for 1 h without a loading dose. The infusion rate was then increased (maximum of 0.7μg·kg·h) or decreased (minimum of 0.2μg·kg·h) every 15 min as deemed clinically necessary by the nurse at the bedside until an RSS between 2 and 4 was achieved. Other sedative/analgesic agents (propofol, midazolam, morphine or fentanyl) were stopped 1 h after dexmedetomidine was started. Nursing staff were instructed to reach the maximum infusion rate of 0.7μg·kg·h before any additional sedatives or analgesia was given, except when the patient had an RSS of 1. Administration of rescue medication was driven by the bedside nurse who assessed the level of sedation using the RSS. Inadequate sedation was treated with an intravenous bolus of 1 mg of midazolam, and inadequate analgesia was treated with an intravenous bolus of 2 mg of morphine or 20μg of fentanyl. For persisting high levels of pain, a continuous infusion was permitted. Minimal additional sedation or analgesia was defined as the use of midazolam of less than 10 mg per 24-h period or the use of less than 10 mg morphine (or 100μg fentanyl) over a 24-h period, respectively. The RSS was obtained hourly for the first 2 h then every 4 h for the duration of the infusion, before and 10 min after any adjustment in study drug infusion rate or the use of rescue medications. No patient received non-steroidal anti-inflammatory drugs. The use of paracetamol was restricted to treatment of hyperpyrexia.
Statistics
All statistical analysis was performed using StatView for Windows (Version 5.0.1 SAS Institute, Cary, N.C., USA). A repeated-measures analysis of variance was used to assess any changes in blood pressures and HR over time.
Results
Over a period of 6 months, 20 patients with a mean age of 61 (±15) years were enrolled. These included 11 medical patients, two multi-trauma patients, and seven complex surgical patients. The mean APACHE II score was 23 (±9) with a median ICU stay of 11 (4–106) days. The median duration of infusion was 71.5 h (35 – 168). The median duration of artificial airway was 7.7 (2.5–105) days. Nine patients had acute renal failure, and five required continuous veno-venous dialytic therapy. Hospital mortality was similar to our unit average (six deaths, 30%). Individual patients’ characteristics including admission diagnosis, co-morbidities, and hospital outcome are shown in Table 1.
Study drug infusion
Dexmedetomidine was started at 0.4μg·kg·h in all patients. As the other sedative/analgesic drugs were withdrawn, there was a steady increase in the dose of study drug infusion with 17/20 patients achieving the maximum allowed infusion rate of 0.7μg·kg·h within the first 36 h.
Ramsay sedation score
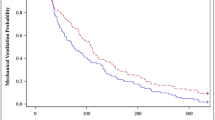
A total of 1,381 observations were obtained over the duration of the infusion in the 20 patients. The target RSS between 2 and 4 was achieved in 926 (67%) of observations. The RSS was between 2 and 5 on 1,147 (83%) occasions. A RSS of 1 was observed in 137 (10%) occasions and a score of 6 was achieved on 97 (7%) occasions (Fig. 1). The observation of an RSS of 6 was reduced from (13%) in the first 6 h to (3%) between the 18-h and 24-h time points. Uneventful extubation for 9 of the 14 survivors was achieved during the infusion of the study drug which continued for a mean of 26 h post-extubation.
Additional sedation
During infusion of dexmedetomidine, 16 of the 20 patients (80%) required minimal or no additional sedation with a median of 4 mg/day (range 0.5–10) of midazolam. The remaining four patients required therapeutic doses of additional sedation with a median midazolam dose of 39 mg/day (range 29 – 41). Two of the four patients had a midazolam infusion introduced 38 h and 128 h into the study when deeper sedation was required to facilitate mechanical ventilation. One patient (patient 18) had a complex clinical state and continued to receive a midazolam infusion from time zero. Medical patients required a median midazolam dose of 6 mg/day (range 0–41) while surgical patients required a median midazolam dose of 3 mg/day (range 0–29).
Additional analgesia
Ten patients needed no or minimal additional analgesia with a median 2 mg/day (range 0.5–4.5) of morphine or 55μg/day (range 14–63) of fentanyl. The ten remaining patients required therapeutic doses of additional analgesia with median morphine/fentanyl doses of 51 mg/day (range 42.5–76) and 599μg/day (range 183–1,098), respectively. Patients who required therapeutic doses of narcotic infusion at the start of the study, continued to need large doses of morphine or fentanyl. These patients were mainly complex surgical or trauma victims. Medical patients required a morphine (or equivalent) dose of 5.5 mg/day (range 0–110) compared to surgical patients 23 mg/day (range 0–89). Five (25%) patients who had renal failure requiring dialysis required a median total infusion dose over the first 24 h of 13.6μg/kg (range 11.8–15.3) versus 9.9μg/kg (range 5.3–16.0) for the non-dialysed patients. Similarly, dialysed patients required higher doses of rescue therapy than all other patients: median morphine equivalents per 24 h was 69 mg (range 6.3–109.8 mg) versus 4.6 mg (range 0–89 mg), and median midazolam dose per 24 h was 28.9 mg (range 0–41.1 mg), versus 3.2 mg (range 0–40.2 mg) (Table 2).
Blood pressure
Mean SBP at baseline was 144 mmHg (±24). This fell to 121 mmHg (±23, 16% <baseline) within 2 h. The lowest single SBP was reported at 12 h, (Fig. 2), after which there was minimal change in SBP (±10%) for any patient throughout the infusion of the study drug (Fig. 3). Patient number 1 had chronic hypertension and developed persistent hypertension requiring intravenous infusion of antihypertensive treatments.
Heart rate
There was a gradual but significant reduction in HR over the first 4 h (ANOVA, repeated measures: f=7.359; P<0.0001) Mean HR was 91 bpm (±23) at baseline and fell continuously over 12 h by 21% to 72 bpm (±14) after which HR showed minimal changes throughout the duration of the infusion.
Inotropes
Nine patients required no inotropes throughout the study period. One patient required noradrenaline (0.03μg·kg·h for 3 h) in addition to volume loading for hypotension within the first 12 h. Five patients were already on noradrenaline, one patient had a slight increase in noradrenaline requirements, and four patients had a reduction in requirements. Two patients were already on dobutamine and the dose of their infusions remained unchanged. Noradrenaline/metaraminol infusion were introduced in two patients and dobutamine in another patient after the first 12 h and continued for longer than 24 h. Overall, eight patients needed inotropic infusions for longer than 24 h. There were no ECG or biochemical changes consistent with myocardial ischaemia or infarction.
Blood pressure and heart rate post-infusion
These were recorded for 24 h after abrupt cessation of study drug and showed minimal changes (Fig. 4). A maximum mean SBP value of 154 mmHg (±24, 7% increase) from a baseline mean of 143 mmHg (±24) was recorded 5 h after cessation. The mean baseline HR at cessation of dexmedetomidine was 86 bpm (±17) and a maximum mean HR of 97 bpm (±17, 11% increase) was observed after 14 h.
Discussion
The role of dexmedetomidine for postoperative sedation and analgesia in patients requiring mechanical ventilation is now well-established [9]; however, most of the patients studied have been elective surgical patients with few comorbidities and do not represent the general intensive care patient.
To test the value of dexmedetomidine as a longer-term sedative in intensive care patients, we adopted a liberal inclusion criterion. All patients who would normally receive standard sedation regardless of severity of illness or organ dysfunction were eligible. This is reflected by the high mean APACHE II score of 23(±9) in our group of patients compared with group described by Venn et al. 2003 [mean APACHE II 16.5(±10)] [8]. Our methodology and patient selection mirrored the sedation practices of many tertiary intensive care units. There were two primary differences between our study and other dexmedetomidine studies; firstly, we eliminated the loading dose; and secondly, the duration of the dexmedetomidine infusion was much longer [mean 81(35–168) h vs 33(13–72) h] in 12 medical ICU patients described by Venn et al. 2003.
In Australian and New Zealand intensive care units sedation practices are generally nurse-driven. Therefore, and in keeping with current clinical practice, the adequacy of sedation and consequent therapeutic intervention is determined by the assessment of the bedside nurse. We were restricted by the current maximum recommended dose of dexmedetomidine (0.7μg·kg·h). Some of our patients may have benefited from a higher dose as shown by a study with a similar patient group where doses of up to 2.5μg·kg·h were used [8].
Most patients had acceptable quality sedation as shown by 83% of observed RSS recorded between 2 and 5 over the study period. An RSS of 1 occurred randomly in 10% of observations over the study period. Similarly, the incidence of over-sedation (RSS of 6) fell from 13% to 3% over the first 24 h. This probably represented a hangover effect from the anaesthesia in the post-operative patients or cumulative sedation from pre-study conventional sedation (see Fig. 1).
Our patients were representative of the very heterogenous mix of tertiary level ICU which led to a marked variation in the need for additional sedation and/or analgesia, influenced by the underlying disease process and its progress. The overall effect on sedation and analgesia was similar in magnitude to studies on postoperative surgical patients with consistent effect [5]. Most patients (16/20) needed minimal or no added sedation. High doses of additional midazolam were required to facilitate the management of deteriorating clinical state in four patients (two patients for ventilatory support and two for severe sepsis). Our medical group required a higher median dose of midazolam for rescue sedation. This was similar to the findings in a group of 12 medical patients who needed either rescue with large doses of propofol or dexmedetomidine infused up to 2.5μg·kg·h [8]. Similarly, our surgical patients needed higher levels of added opioid than medical patients. The need for high doses of additional narcotics was manifested within 12 h and continued throughout the infusion. This was particularly true for the ten patients who required high doses of additional analgesia. Patients who received dialysis required a higher infusion dose and there was a greater need for rescue medication when compared to patients with normal renal function. The relatively small volume of distribution (137 l) of dexmedetomidine and the lipophilic nature [10] of the molecule may enhance its clearance with continuous dialytic therapy.
To date, most current practices involve weaning any sedation prior to extubation. Dexmedetomidine, due to its lack of ventilatory depression [6, 11], offers a unique opportunity of continuing sedation throughout the process of ventilator weaning and subsequent extubation. It was very encouraging that 65% of the surviving patients were extubated whilst dexmedetomidine was still being infused.
A unique feature of our study was the omission of dexmedetomidine loading dose. Venn et al. demonstrated that of 66 patients receiving dexmedetomidine with a loading dose, nine out of 11 hypotensive events occurred during the loading dose period [5]. In a study of medical patients [8] the same author recommended a reduction in loading dose to avoid hypotension during this phase describing significant hypotension and bradycardia with other patients experiencing hypertension during loading dose [5, 8]. A predictable decrease in blood pressure and HR was observed in our patients; however, onset was slower, less pronounced, and more delayed when compared with other studies that used a loading dose [4, 5]. Only one patient needed a minimal short-lived intervention (0.03μg·kg·min noradrenaline for 3 h) after commencement of the study drug. All other interventions were dictated by the underlying disease process and introduced more than 12 h after study induction.
Rebound phenomena is a complex process well-known after the use of antihypertensive drugs and, in particular, after the cessation of centrally acting α-2-receptor agonists [12]. Well-described rebound hypertension from clonidine is associated with sympathetic overactivity and high plasma levels of noradrenaline and adrenaline [7, 8, 13]. There is no corresponding data regarding rebound hypertension and tachycardia after abrupt cessation of dexmedetomidine. Venn and colleagues [6] showed no evidence of rebound in patients receiving dexmedetomidine for up to 24 h. In this study, dexmedetomidine infusion was ceased (not weaned) once sedation was no longer necessary. In our study, there was minimal increase in blood pressure and HR (7% and 11% maximum rise, respectively) up to 24 h after cessation. This may be explained by the high alpha-2 receptor selectivity or because of the continuous infusion and modestly long terminal half-life of dexmedetomidine of 2–3 h that allows a slow recovery of the sympathetic system. Interestingly, sustained-release clonidine patches studied in more than 2,000 patients resulted in no evidence of rebound upon discontinuation. Additionally, there were no reported clinical symptoms or signs to suggest a withdrawal syndrome [14].
Although this was an observational study, it was designed to reflect the complexity of heterogeneous critically ill patients. The effectiveness of sedative drugs in this group of patients may be influenced by underlying co-morbidities making effective evaluation difficult. The nature of a nurse-driven sedation may introduce distortion through subjective assessment of sedation or the use of additional therapy. There are inherent limitations in the design of observational studies; in particular, the small group size of our study and the absence of a control group. Similarly, the unblinded nature of this study raises the potential for bias. Therefore, our results cannot be generalised until validated by properly conducted randomised control trials.
Dexmedetomidine appears to have a promising potential as an effective sedative agent in critically ill patients and can be used safely for up to 7 days, with stable and predictable haemodynamic effects on induction and cessation. Although inadequate for some patients, a licensed dose of up to 0.7μg·kg·h offers a significant reduction in other sedation requirements and is sparing of narcotic needs.
References
Kress JP, Pohlman AS, O’Connor MF, Hall JB (2000) Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. New Engl J Med 342:1471–1477
Bhana N, Goa KL, McClellan KJ (2000) Dexmedetomidine. Drugs 59:263–268
Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, Heard S, Cheung A, Son SL, Kallio A (2000) The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg 90:834–839
Triltsch AE, Welte M, von Homeyer P, Grosse J, Genahr A, Moshirzadeh M, Sidiropoulos A, Konertz W, Kox WJ, Spies CD (2002) Bispectral index-guided sedation with dexmedetomidine in intensive care: a prospective, randomized, double blind, placebo–controlled phase II study. Crit Care Med 30:1007–10014
Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, Vedio A, Singer M, Feneck R, Treacher D, Willatts SM, Grounds RM (1999) Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia 54:1136–1142
Venn RM, Hell J, Grounds RM (2000) Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care (London) 4:302–308
Venn RM, Grounds RM (2001) Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions. Br J Anaesth 87:684–690
Venn M, Newman J, Grounds M (2003) A phase II study to evaluate the efficacy of dexmedetomidine for sedation in the medical intensive care unit. Intensive Care Med 29:201–207
Herr DL, Sum-Ping ST, England M (2003) ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesthes 17:576–584
Venn RM, Karol MD, Grounds RM (2002) Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive care. Br J Anaesth 88:669–775
Belleville JP, Ward DS, Bloor BC, Maze M (1992) Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology 77:1125–1133
Cummings DM, Vlasses PH (1982) Antihypertensive drug withdrawal syndrome. Drug Intel Clin Pharm 16:817–8122
Geyskes GG, Boer P, Dorhout Mees EJ (1979) Clonidine withdrawal. Mechanism and frequency of rebound hypertension. Br J Clinical Pharmac 7:55–62
Lowenthal DT, Saris S, Paran E, Cristal N, Sharif K, Bies C, Fagan T (1986) Efficacy of clonidine as transdermal therapeutic system: the international clinical trial experience. Am Heart J 112:893–900
Acknowledgments
We thank David Bihari and the intensive care nursing staff for their contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was performed in the Intensive Care Units of the Prince of Wales hospital, a principal teaching hospital of the University of New South Wales and the Prince of Wales private hospital. Abbott Australia provided the study drug free of charge
Rights and permissions
About this article
Cite this article
Shehabi, Y., Ruettimann, U., Adamson, H. et al. Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects. Intensive Care Med 30, 2188–2196 (2004). https://doi.org/10.1007/s00134-004-2417-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2417-z