Abstract
The extracellular matrix (ECM) plays an important role in the biomechanical behaviour of the lung parenchyma. The ECM is composed of a three-dimensional fibre mesh filled with different macromolecules, including the glycosaminoglycans and the proteoglycans, which have important functions in many lung pathophysiological processes: (1) regulating the hydration and water homeostasis, (2) maintaining the structure and function, (3) modulating the inflammatory response, and (4) influencing tissue repair and remodelling. Ventilator-induced lung injury is the result of a complex interplay among various mechanical forces acting on lung structures such as the epithelial and endothelial cells, the extracellular matrix, and the peripheral airways during mechanical ventilation. Although excellent reviews have synthesized our current knowledge of the role of repeated cyclic stretch and high tidal volume ventilation on alveolar and endothelial cells, few have addressed the effects of mechanical ventilation on the ECM. The present review focused on the organization of the ECM, mechanotransduction and ECM interactions, and the effects of mechanical ventilation on the ECM. The study of the ECM may be useful to improve our understanding of the pathophysiology of lung damage induced by mechanical ventilation.
Similar content being viewed by others
Introduction
Mechanical ventilation may worsen pre-existing lung disease due to inhomogeneous distribution of insufflated air, resulting in regional overdistension (volutrauma and barotrauma) and derecruitment, which leads to repeated opening and closing of collapsed alveoli (atelectrauma) [1, 2]. Ventilator-induced lung injury (VILI) is the result of a complex interplay among various mechanical forces acting on lung structures during mechanical ventilation [2]. Critical physical forces contributing to VILI have been defined as “stress” (force per unit of area) or “strain” (force along longitudinal axis) [3], and their primary possible targets include: (1) the epithelial [4] and (2) endothelial [5] cells, (3) the extracellular matrix (ECM) [6, 7], and (4) the peripheral airways [8, 9].
Although excellent reviews have synthesized our current knowledge of the role of repeated cyclic stretch and high tidal volume ventilation on alveolar and endothelial cells, few have addressed the effects of mechanical ventilation on the ECM.
In this review, we will discuss: (a) the organization of the ECM; (b) mechanotransduction and ECM interactions; and (c) the effects of mechanical ventilation on the ECM.
Extracellular matrix organization
The ECM is not only a scaffold, having a mechanical role in supporting and maintaining tissue structure, but is also a complex and dynamic meshwork influencing many biological cell functions such as lung development, cell migration, and proliferation. The macromolecules that constitute the ECM are: (1) fibrous proteins (collagen and elastin); (2) structural or adhesive proteins (fibronectin and laminin); and (3) proteoglycans and glycosaminoglycans (Fig. 1). In addition, matrix metalloproteinases play a relevant role at maintaining the turnover of ECM molecules.
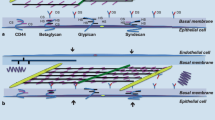
Extracellular matrix components in lung parenchyma. Note the predominance of versican in the pulmonary interstitium, perlecan in the vascular basement membrane, and decorin in the interstitium and in the epithelial basement membrane linked with collagen fibrils, syndecan, and glypican on the cell surface. CS, Chondroitin sulphate; HS, heparan sulphate; DS, dermatan sulphate
Collagen
Collagen fibre constitutes the main component of ECM. The most important collagen fibres are: types I, II, III (fibrillar) and IV, V, VI (non-fibrillar). The turnover of collagen fibres is a dynamic process, necessary for the maintenance of the normal lung architecture [10]. The amount of collagen deposition depends on the extent of the alveolar injury and the intensity of inflammatory mediator release in the lung parenchyma [11]. Type III collagen fibre, which is more flexible and susceptible to breakdown, predominates early in the course of lung injury, whereas type I collagen (comprised of thicker and cross-linked fibrils) is more prevalent in the late phase [12, 13].
Elastin
Elastic fibres comprise three components defined according to their amount of elastin and fibril orientation: (1) oxytalan, composed of a bundle of microfibrils; (2) elaunin, composed of microfibrils and a small amount of elastin; and (3) fully developed elastic fibres, composed of microfibrils and abundant elastin [10]. Chondroblasts, myofibroblasts, and smooth muscle cells synthesize these fibres. Due to their mechanical properties, elastic fibres provide recoil tension to restore the parenchyma to its previous configuration after the stimulus for inspiration has ceased. In normal alveolar septa, a subepithelial layer of elastic fibres composed mainly of fully mature elastic fibres confers great elasticity to the alveolar tissue in normal situations [10]. The elastic component of the ECM represents one of the structures potentially involved in alveolar remodelling as well as in the consequent loss of pulmonary compliance observed in ARDS [11].
Glycosaminoglycans and proteoglycans
In the connective tissue, proteoglycans (PGs) form a gelatinous and hydrated substance embedding the fibrous proteins. Proteoglycans are constituted of a central protein bound to one or more polysaccharides, denominated glycosaminoglycans (GAGs).
Glycosaminoglycans
GAGs are long, linear, and heterogeneous polysaccharides that consist of repeating disaccharide units. There are two main types of GAGs: (1) non-sulphated GAG (hyaluronic acid) and (2) sulphated GAGs (heparan sulphate and heparin, chondroitin sulphate, dermatan sulphate, and keratan sulphate). With the exception of hyaluronic acid, GAGs are usually covalently attached to a protein core, forming an overall structure referred to as proteoglycan [14].
Non sulphated glycosaminoglycan (hyaluronic acid): Hyaluronic acid is the most abundant and the largest non-sulphated GAG in the ECM. It is primarily synthesized by mesenchymal cells, being a necessary molecule for the assembly of a connective tissue matrix and an important stabilizing constituent of the loose connective tissue. Hyaluronic acid is an important determinant of tissue hydration [15] and is also involved in tissue repair [16] and in protection against infections and proteolytic granulocyte enzymes [17].
Sulphated glycosaminoglycans: These other GAGs are synthesized intracellularly, sulphated, secreted, and usually covalently bound to a protein core to form the proteoglycans. The most important sulphated GAGs are chondroitin sulphate, heparan sulphate, and dermatan sulphate. The polyanionic nature of GAGs is the main determinant of the physical properties of proteoglycan molecules, allowing them to resist compressive forces and to simultaneously maintain tissue hydration [18].
Proteoglycans
In the lung, the main proteoglycan families may be distinguished based on GAGs' composition, molecular weight, and function: chondroitin-sulphate-containing proteoglycan (versican), heparan-sulphate-containing proteoglycans (perlecan and glypican), chondroitin- and heparan-sulphate-containing proteoglycan (syndecan), and dermatan-sulphate-containing proteoglycan (decorin). They are localized in different areas of the ECM: versican in the pulmonary interstitium; perlecan in the vascular basement membrane; decorin in the interstitium and in the epithelial basement membrane linked with collagen fibrils; syndecan and glypican at the cell surface [19] (Fig. 1).
Proteoglycans have a number of different biological functions. Versican, due to the high ionic charge of its multiple GAG side-chains, plays a critical role in determining the water content or turgor of extracellular matrices, influencing tissue viscoelastic behaviour as well as cell migration and proliferation. Additionally, perlecan acts as a filtration barrier [20] and syndecan functions primarily as a cell surface receptor for matrix ligands [21]. Decorin and biglycan bind to collagen and affect fibrinogenesis and matrix assembly [22]. Proteoglycans also bind various growth factors, such as transforming growth factor (TGF)-β and fibroblast growth factor (FGF), and modulate their effect on cell proliferation and matrix deposition by influencing their bioavailability [23].
Basal lamina molecules
The basal lamina of pulmonary cells is composed of different molecules, including like laminin, nidogen, and perlecan [24]: (1) Laminin is a long sword-shaped trimer [25]. The end of the “sword” can bind cell receptors, and the crosspieces allow laminin to bind to other laminin molecules. Other sites for nidogen and perlecan binding are also present in the molecule. (2) Nidogen, also known as entactin, bridges between the laminin and collagen layers and perlecan in the basal lamina. (3) Perlecan is the predominant proteoglycan in the basal lamina. It binds collagen, laminin, itself, and nidogen [26] (Fig. 1).
On one side of the cell membrane, the basal lamina is linked to the cell by means of integrins [27, 28], while on the other side it is liked to the ECM through links to collagen type IV. Integrins are adhesive membrane receptors that exist as heterodimers. They exhibit a “Velcro” effect: they have strength in numbers, but are individually easy to disrupt. They require Ca2+ or Mg2+ to bind, and their job is to link the ECM to the cytoskeleton. Fibronectin reinforces the connections between the basal lamina with both the cell membrane and the other ECM components.
Extracellular matrix metalloproteinases
Matrix metalloproteinases (MMPs) are a family of enzymes that degrade components of the ECM, including collagens, fibronectin, laminin, proteoglycans, entactins, and elastin [29]. In particular, they are very important in: (a) the breakdown of ECM and basement membrane, (b) tissue remodelling and angiogenesis, and (c) the restoration of functional connective tissue in the wound-healing process.
The 72-kDa gelatinase A (MMP-2), is the most widely distributed of all the MMPs and is expressed constitutively by a number of cells, including endothelial and epithelial cells. The 92-kDa gelatinase B (MMP-9) is produced by several types of inflammatory cells, including PMNs and alveolar macrophages, as well as stimulated connective tissue cells. Gelatinase A, along with gelatinase B, plays an important role in pericellular basement membrane turnover by degrading type IV collagen, a main component of the basement membrane [30].
Because MMPs may cause significant host damage, their proteolytic activity is tightly regulated. Thus, MMPs are rarely stored, but require gene transcription before secretion, with the exception of neutrophil MMP-9. In fact, these are either secreted as pro-enzymes that require proteolytic cleavage or are activated intracellularly by pro-protein convertases such as furin. Specific inhibitors of MMPs, the tissue inhibitors of metalloproteinases (TIMPs), which bind MMPs in a 1:1 manner, are secreted to prevent enzymatic activity; as a result, the balance between MMPs and TIMPs determines matrix turnover, where either an excess of MMPs or a deficit of TIMPs may result in excess ECM degradation [30].
The majority of MMPs are not expressed in normal healthy tissues, but are expressed in diseased tissues that are inflamed or undergoing repair and remodelling [31]. MMP expression may be upregulated by exogenous stimuli, cytokines, and cell–cell contact. Conversely, cytokines such as interferon-γ and interleukins (IL)-4 and -10 may downregulate MMP expression. Both inflammatory and stromal cells can express MMPs, although the profile is both cell and stimulus specific.
Mechanotransduction and the ECM
Mechanotransduction is the conversion of mechanical stimuli into biochemical and biomolecular signals [32, 33]. The mechanosensor is defined as the system that detects the mechanical stimuli on the cell and converts it into a biological signal [34]. While much has been elucidated regarding the ability of pulmonary cells to sense and integrate information from mechanical distortion, little is known about how this information alters the ECM and affects outcome in patients on mechanical ventilation.
Living cells often need to integrate biochemical signals with mechanical information from their microenvironment [35]. This mechanotransduction is powerful, eliciting proliferation, differentiation, or apoptosis in a manner dependent upon the extent of physical deformation. The cell's internal “pre-stressed” structure and its “hardwired” interaction with the ECM appear to confer the ability to filter biochemical signals and decide between divergent cell functions influenced by the nature of signals from the mechanical environment. In some instances mechanical signalling through the tissue microenvironment has been shown to be dominant over genomic signals. Indeed, mechanical interactions between cells and the matrix are known to modulate cell contractility and myosin light chain phosphorylation [36], cell rheology [37], and focal adhesion assembly [38], all of which are critical for the control of cell adhesion, migration, growth, contractility, and viability. Additionally, the mechanical properties of the ECM may influence angiogenesis [39], as well as connective tissue homeostasis itself [40]. The direct interaction between the ECM and cellular biochemistry also has important implications for the biomechanical properties of the connective tissues. Consequently, when we discuss the pathological effects of mechanical force on the ECM, it is also fundamental that we keep in mind the “normal” and beneficial role of cyclic stretch on lung form and function.
Mechanosensing by the ECM
Virtually all organs and tissues are organized as pre-stressed structural hierarchies that exhibit immediate mechanical responsiveness and increase their stiffness in direct proportion to the applied mechanical stress [27]. In the lung, the residual filling pressure that remains after expiration is responsible for: (1) tensing and stiffening the ECMs (basement membranes, collagen, elastin) that surround each alveolus, and (2) resisting surface tension forces acting on the epithelium [41]. Lung expiration and inspiration influence this force balance and produce complex micromechanical responses in the lung parenchyma, including lengthening and shortening (and tension and compression) of alveolar walls depending on the direction of the applied stress [42, 43]. This is accompanied by extension and linearization of some collagen fibres on inspiration, as well as buckling of the same fibres on expiration. Breathing also causes the lateral intercellular spaces between epithelial cells to reversibly shrink and expand without compromising the structural integrity of the tissue. This form of reversible mechanical deformation might activate intracellular signalling within surrounding alveolar cells by altering the local concentration of soluble ligands for epidermal growth factor receptors [28]. Other systems have developed alternative mechanosensing strategies that differ markedly from the ones presumed to be functional in the lung. In cartilage, for example, changes in ion concentrations in the surrounding interstitium can influence whole tissue mechanics by altering the swelling of proteoglycans and consequently the pressure in the cartilage [44].
Mechanotransduction and integrins
The leading hypothesis regarding how the ECM senses mechanical force has been proposed by Ingber [45]. Based on the tensegrity model, the proposal is that the interstitium senses mechanical forces via the integrin adhesion receptors that connect the cytoskeleton to the ECM. Cells sense distortion of the ECM, or an associated increase in its rigidity, as a tug on these adhesion receptors. Integrins connect to the cytoskeleton through focal adhesions that contain multiple actin-associated proteins such as talin, vinculin, paxillin, and zyxin (Fig. 2). The cytoskeleton, in turn, responds mechanically to forces transferred over the ECM and channelled through integrins by rearranging its interlinked actin microfilaments, microtubules, and intermediate filaments, as well as associated organelles (e. g., mitochondria) and nuclei, thereby strengthening the whole cell against the potential deleterious effects of mechanical distortion [27]. The ability to channel mechanical forces over discrete molecular paths to sites deep inside the cytoplasm and nucleus explains how cell distortion or mechanical stress application to ECM and bound cell surface integrins result in predictable and focused changes in nuclear activity and, hence, biomolecular changes in cell form and function.
Mechanotransduction and laminin
In addition to collagen, integrin receptors can also anchor to other ECM molecules such as fibronectin or laminin. Recently, Jones and colleagues [25] demonstrated a novel mechanism for mechanotransduction in type II alveolar cells. These cells secrete ECM rich in anastomosing fibres composed of the α3 laminin subunits (i. e. laminin 6), and perlecan. Using an in vitro model, cyclic stretch (30 cycles/min, 10% strain) induced activation of mitogen-activated protein kinase (MAPK) that could not be inhibited by treatment with an antagonist to the α3 laminin subunit (function-inhibiting antibody) (Fig. 2). In contrast, when dystroglycan is knocked down using short hairpin RNA, MAPK activation is inhibited. These results support the hypothesis that, in addition to integrins, signals generated by mechanical stretch could be internalized by laminin-6, via interaction with dystroglycan. Stretching deflects matrix fibres that transduce signals via dystroglycan on the surface of alveolar epithelial cells. Further studies are required to determine whether the choice of different mechanisms represent a cell- or dose-specific response and how this can be integrated at tissue level.
Mechanotransduction and proteoglycans
Collagen and elastin are thought to dominate the elasticity of the connective tissue, including lung parenchyma. The GAGs or the proteoglycans may also play a role because osmolarity of interstitial fluid can alter the repulsive forces on the negatively charged GAGs, allowing them to collapse or inflate, which can affect the stretching and folding pattern of the fibres. Hence, the elasticity of lung has been hypothesized to arise primarily from: (1) the topology of the collagen–elastin network and (2) the mechanical interaction between proteoglycans and fibres. More recently, it has also been suggested that proteoglycans may play a fundamental role in stabilizing the collagen–elastin network of connective tissue and contributing to lung elasticity and alveolar stability at medium lung volumes [46].
Further studies are required to understand how signals generated by proteoglycan swelling may lead to this process and the pathophysiology of VILI.
Impact of mechanical ventilation on ECM
Mechanical forces can modify the gene expression of several molecules related to the inflammatory [47–49] and remodelling [6, 50–53] processes. These forces induce direct secretion of various growth factors, such as TGF-β and connective tissue growth factor (CTGF), which accelerate the remodelling of the matrix and extracellular protein release, respectively [28, 54–56]. Because large amounts of TGF-β are present in both the alveoli and airways of healthy adults, much of the regulation of TGF-β occurs at the level of activation of stored latent complexes. In the alveoli, much of this activation appears to be controlled through spatially restricted activation by the integrin α-v-β-6, but the mechanisms regulating TGF-β activity in the conducting airways remain to be determined [56]. Integrin expressed on the luminal surface can present active TGF-β to luminal macrophages, thereby inhibiting protease secretion and maintaining alveolar homeostasis. Integrin on the basal surface can present active TGF-β to fibroblasts, which in excess contributes to the development of pulmonary fibrosis and also to endothelial cells, which regulate pulmonary vascular permeability. Integrin on the lateral surface can present active TGF-β to adjacent epithelial cells, increasing epithelial permeability and decreasing the reabsorption of salt, and therefore water, from the alveolar space. TGF-β directly increases the permeability of both pulmonary endothelial and alveolar epithelial monolayers [57]. Furthermore, TGF-β leads to a dramatic reduction in the expression of the sodium channel ENaC on the apical surface of alveolar epithelial cells [58], thereby impairing the removal of salt and water from the alveolar lumen. Thus, it appears that activation of TGF-β by the α-v-β-6 integrin plays an essential role in adjusting the set point for the amount of active TGF-β at specific sites in the lung parenchyma, limiting damage caused by unrestricted inflammation as well as promoting alveolar repair.
High-volume ventilation initiates ECM remodelling in patients [59] and experimental models [6, 50, 52, 53, 60]. This event depends on an airway pressure gradient [6, 60] and a transpleural pressure gradient. Berg and colleagues [60] observed higher levels of mRNA for α1 [III] and α2 [IV] procollagen, fibronectin, FGF, and TGF-β1 in lungs ventilated with high PEEP levels. In this regard, Parker and colleagues [6] found that ventilation with high peak airway pressures and low perfusion pressures led to greater type III procollagen mRNA expression than in unperfused lungs. Type III procollagen is the first collagen to be remodelled in the evolution of lung fibrogenesis and has been used as an early marker of lung parenchyma remodelling [6, 50, 52, 53]. Garcia and colleagues [50] demonstrated that the increase in the tissue stress induced by oscillation force, but not amplitude, increases procollagen type III mRNA expression in rat lung parenchymal strips. In addition, they observed that there is a threshold stress above which lung cells express mRNA for type III procollagen. Furthermore, Farias and colleagues [53] showed that even a short period (40 s) of lung mechanical stretch (40 cmH2O of continuous positive airway pressure) increased type III procollagen mRNA expression. Along these lines, De Carvalho and colleagues reported that type III procollagen expression was higher in non-dependent regions and in ventilatory strategies that caused overdistension, and this response was partially attenuated by prone positioning [52]. In fact, in healthy rat lungs submitted to injurious ventilation either with high or low tidal volume (VT), ECM reacted with an increased synthesis of mRNA for procollagen type III, which was more pronounced in non-dependent regions of the lungs [61]. This suggests an effect of regional transpleural forces that emerged due to lung heterogeneity in the context of VILI. Furthermore, high VT leads to an increase in expression and release of gelatinases from epithelial and endothelial cells in the lung due to the mechanical stress on these cells. There is increasing evidence that cyclic mechanical stress affects the release and activation of MMPs and plays an important role in the regulation of ECM remodelling. Cyclic mechanical stress causes the activation of human alveolar macrophages in vitro and their release of gelatinase B [62]. Moreover, Foda and colleagues, in an in vivo rat model of high-volume ventilation, observed that MMPs play an important role in the development of VILI [63].
Mechanical strain also leads to modifications in proteoglycans and GAGs. Al Jamal et al. showed in a model of high VT ventilation in healthy rats [7] that, at the highest VT, the expression of versican, heparan-sulphate proteoglycan, and biglycan in lung tissue was increased. In addition, Moriondo and colleagues [64] observed heparan sulphate fragmentation at high VT, suggesting ventilation-induced plasma membrane disruption. Altered proteoglycans may also contribute to the pathology seen in response to excessive ventilation through their putative pro-inflammatory effects (Fig. 3). GAGs have shown highly specific interactions with various chemokines, such as CCL5/RANTES, CCL2, MCP-1, and CXCL8/IL-8 [65]. In addition, PGs may act as ligands for pro-inflammatory Toll-like receptors [66]. Therefore, fragmentation of GAGs and breakdown of proteoglycans may have an impact on the development of the inflammatory response seen in VILI.
Changes in extracellular matrix during spontaneous breathing and mechanical ventilation (MV) with normal or high tidal volume (V T ). In mechanical ventilation at normal tidal volume (6–8 ml/kg) an initial fragmentation of both heparan sulphate (HS) and chondroitin sulphate (CS) proteoglycans is triggered by the activation of a few metalloproteinases (grey discs). Deeper degradation of both heparan sulphate and chondroitin sulphate proteoglycans and loss of the entire ECM structure and function is observed in mechanical ventilation at high VT. At normal VT, matrix breakdown is associated with enhanced metalloproteinases' degradative digestion and is not associated with release of inflammatory mediators. W/D, Wet-to-dry weight ratio
Additionally, the concept of “matrikines” may be considered. Matrikines are a new class of ligands which exist as a domain within an ECM protein. Natural matrikines are those which signal directly from the ECM, while cryptic matrikines are those that require proteolytic breakdown for the ligand to be revealed [67]. Decorin is an example of a proteoglycan matrikine; it functions through the EGF receptor and activates downstream signalling pathways, such as ERK1/ERK2 [68]. Thus, breakdown of ECM proteins caused by high VT results in an exposure of matrikines, which can then act as ligands to induce subsequent biologic effects [69].
Recent animal studies demonstrated that mechanical ventilation with low VT may also cause histological damage to peripheral airways and interstitium in healthy lungs [8, 70–72]. These alterations are most likely the consequence of abnormal stresses that develop locally at the level of both the bronchiolar epithelium and the parenchyma, mainly at the alveolar–bronchiolar junctions, because of the cyclic opening and closing of peripheral airways at low end-expiratory lung volume [73]. Duggan and colleagues showed ultrastructural evidence of microvascular endothelial disruption during ventilation with VT of 8 ml/kg and respiratory frequency of 40 breaths per minute [74]. Interestingly, microvascular injury in their model was more pronounced in animals that did not receive recruitment manoeuvre, suggesting that repeated cyclic stretch of collapsed alveoli, rather than overdistended ones, may play a critical role in injury to pulmonary structures. Moriondo and colleagues [64] reported marked fragmentation of the interstitial and basal membrane proteoglycans in anaesthetized rats ventilated for 4 h at 7 ml/kg VT. In contrast, other studies have shown that low-VT ventilation does not adversely affect the ECM. Al Jamal and colleagues [7] did not find an increase in synthesis of proteoglycans in the ECM after 1 h of mechanical ventilation with VT of 8 ml/kg. Farias and colleagues [53] reported that 1 h of ventilation with 6 ml/kg VT in healthy rat lungs did not increase type III procollagen mRNA expression. An important question is whether these alterations are mainly due to activation of an inflammatory process or to direct mechanical damage associated with an ultrastructural lesion (Fig. 3).
Conclusions
Mechanical ventilation may induce physical forces such as stress and/or strain acting on different structures: epithelium, endothelium, peripheral airways, and extracellular matrix. Although the role of repeated cyclic stretch and high VT and pressure on alveolar and endothelial cells are well studied, the impact of mechanical ventilation on the ECM needs to be unveiled. Mechanical ventilation affects the macromolecules that constitute the ECM (collagen, elastin, fibronectin, laminin, proteoglycan, and glycosaminoglycans) which suffer changes and impact the biomechanical behaviour of the lung parenchyma. Furthermore, changes in ECM alter the mechanical forces on the cells, influencing the way that cells remodel the interstitium. In this regard, the reduction in tidal volume or transpulmonary pressure is associated with a more uniform distribution of the stress and strain with less ECM disorganization, diminishing the risk of rupture and remodelling. The study of the ECM may help to improve our understanding of the pathophysiology of lung damage induced by mechanical ventilation.
References
Dreyfuss D, Soler P, Basset G, Saumon G (1988) High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 137:1159–1164
Dos Santos CC, Slutsky AS (2006) The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol 68:585–618
Vlahakis NE, Hubmayr RD (2005) Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med 171:1328–1342
Budinger GR, Sznajder JI (2006) The alveolar–epithelial barrier: a target for potential therapy. Clin Chest Med 27:655–669
Marini JJ, Hotchkiss JR, Broccard AF (2003) Bench-to-bedside review: microvascular and airspace linkage in ventilator-induced lung injury. Crit Care 7:435–444
Parker JC, Breen EC, West JB (1997) High vascular and airway pressures increase interstitial protein mRNA expression in isolated rat lungs. J Appl Physiol 83:1697–1705
Al Jamal R, Ludwig MS (2001) Changes in proteoglycans and lung tissue mechanics during excessive mechanical ventilation in rats. Am J Physiol Lung Cell Mol Physiol 281:L1078–L1087
D'Angelo E, Pecchiari E, Baraggia P, Saetta M, Balestro E, Milic-Emili J (2002) Low-volume ventilation causes peripheral airway injury and increased airway resistance in normal rabbits. J Appl Physiol 92:949–956
Jain M, Sznajder JI (2007) Bench-to-bedside review: Distal airways in acute respiratory distress syndrome. Crit Care 11:206
Montes GS (1996) Structural biology of the fibres of the collagenous and elastic systems. Cell Biol Int 20:15–27
Rocco PRM, Negri EM, Kurtz PM, Vasconcellos FP, Silva GH, Capelozzi VL, Romero PV, Zin WA (2001) Lung tissue mechanics and extracellular matrix in acute lung injury. Am J Respir Crit Care Med 164:1067–1071
Rocco PRM, Souza AB, Faffe DS, Passaro CP, Santos FB, Negri EM, Lima JGM, Contador RS, Capelozzi VL, Zin WA (2003) Effect of corticosteroid on lung parenchyma remodeling at an early phase of acute lung injury. Am J Respir Crit Care Med 168:677–684
Santos FB, Nagato LKS, Boechem NM, Negri EM, Guimarães A, Capelozzi VL, Faffe DS, Zin WA, Rocco PRM (2006) Time course of lung parenchyma remodeling in pulmonary and extrapulmonary acute lung injury. J Appl Physiol 100:98–106
Souza-Fernandes AB, Pelosi P, Rocco PR (2006) Bench-to-bedside review: the role of glycosaminoglycans in respiratory disease. Crit Care 10:237
Tammi MI, Day AJ, Turley EA (2002) Hyaluronan and homeostasis: a balancing act. J Biol Chem 277:4581–4584
Li Y, Rahmanian M, Widstrom C, Lepperdinger G, Frost GI, Heldin P (2000) Irradiation induced expression of hyaluronan (HA) synthase 2 and hyaluronidase 2 genes in rat lung tissue accompanies active turnover of HA and induction of types I and III collagen gene expression. Am J Resp Cell Mol Biol 23:411–418
Cantor JO, Shteyngart B, Cerreta JM, Liu M, Armand G, Turino GM (2000) The effect of hyaluronan on elastic fiber injury in vitro and elastase-induced airspace enlargement in vivo. Proc Soc Exp Biol Med 225:65–71
Hardingham T, Fosang AJ (1992) Proteoglycans: many forms and many functions. FASEB J 6:861–870
Roberts CR, Wight TN, Hascall VC (1997) Proteoglycans. In: Crystal RG, West JB, Weibel ER, Barnes PJ (eds) The lung, 2nd edn. Scientific Foundations. Lippincott–Raven, Philadelphia, pp 757–767
Murdoch AD, Dodge GR, Cohen I, Tuan RS, Iozzo RV (1992) Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Biol Chem 267:8544–8557
Maniscalco WM, Campbell MH (1992) Alveolar type II cells synthesize hydrophobic cell-associated proteoglycans with multiple core proteins. Am J Physiol 263:L348–L356
Geng Y, McQuillan D, Roughley PJ (2006) SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol 25:484–491
Ruoslahti E, Yamaguchi Y (1991) Proteoglycans as modulators of growth factor activities. Cell 64:867–869
Furuyama A, Mochitate K (2000) Assembly of the exogenous extracellular matrix during basement membrane formation by alveolar epithelial cells in vitro. J Cell Sci 113:859–868
Jones JC, Lane K, Hopkinson SB, Lecuona E, Geiger RC, Dean DA, Correa-Meyer E, Gonzales M, Campbell K, Sznajder JI, Budinger S (2005) Laminin-6 assembles into multimolecular fibrillar complexes with perlecan and participates in mechanical-signal transduction via a dystroglycan-dependent, integrin-independent mechanism. J Cell Sci 118:2557–2566
Nguyen NM, Bai Y, Mochitate K, Senior RM (2002) Laminin alpha-chain expression and basement membrane formation by MLE-15 respiratory epithelial cells. Am J Physiol Lung Cell Mol Physiol 282:L1004–L1011
Ingber DE (2006) Cellular mechanotransduction: putting all the pieces together again. FASEB J 20:811–827
Tschumperlin DJ, Dai G, Maly IV, Kikuchi T, Laiho LH, McVittie AK, Haley KJ, Lilly CM, So PT, Lauffenburger DA, Kamm RD, Drazen JM (2004) Mechanotransduction through growth-factor shedding into the extracellular space. Nature 429:83–86
Greenlee KJ, Werb Z, Kheradmand F (2007) Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev 87:69–98
Elkington PT, Friedland JS (2006) Matrix metalloproteinases in destructive pulmonary pathology. Thorax 61:259–266
Lanchou J, Corbel M, Tanguy M, Germain N, Boichot E, Theret N, Clement B, Lagente V, Malledant Y (2003) Imbalance between matrix metalloproteinases (MMP-9 and MMP-2) and tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in acute respiratory distress syndrome patients. Crit Care Med 31:536–542
Liu M, Tanswell AK, Post M (1999) Mechanical force-induced signal transduction in lung cells. Am J Physiol 277:L667–L683
Han B, Lodyga M, Liu M (2005) Ventilator-induced lung injury: role of protein–protein interaction in mechanosensation. Proc Am Thorac Soc 2:181–187
Uhlig S (2002) Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol 282:L892–L896
Giancotti FG, Ruoslahti E (1999) Integrin signaling. Science 285:1028–1032
Polte TR, Eichler GS, Wang N, Ingber DE (2004) Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am J Physiol Cell Physiol 286:518–528
Rosenblatt N, Hu S, Chen J, Wang N, Stamenovic D (2004) Distending stress of the cytoskeleton is a key determinant of cell rheological behavior. Biochem Biophys Res Commun 321:617–622
Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE (2003) Cell shape provides global control of focal adhesion assembly. Biochem Biophys Res Commun 307:355–361
Ingber DE (2002) Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91:877–887
Chiquet M, Renedo AS, Huber F, Fluck M (2003) How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol 22:73–80
Stamenovic D (1990) Micromechanical foundations of pulmonary elasticity. Physiol Rev 70:1117–1134
Toshima M, Ohtani Y, Ohtani O (2004) Three-dimensional architecture of elastin and collagen fiber networks in the human and rat lung. Arch Histol Cytol 67:31–40
Brewer KK, Sakai H, Alencar AM, Majumdar A, Arold SP, Lutchen KR, Ingenito EP, Suki B (2003) Lung and alveolar wall elastic and hysteretic behavior in rats: effects of in vivo elastase treatment. J Appl Physiol 95:1926–1936
Lai WM, Hou JS, Mow VC (1991) A triphasic theory for the swelling and deformation behavior of articular cartilage. J Biomech Eng 113:245–258
Ingber D (1991) Integrins as mechanochemical transducers. Curr Opin Cell Biol 3:841–848
Cavalcante FS, Ito S, Brewer K, Sakai H, Alencar AM, Almeida MP, Andrade JS, Majumdar A, Ingenito EP, Suki B (2005) Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol 98:672–679
Copland IB, Kavanagh BP, Engelberts D, McKerlie C, Belik J, Post M (2003) Early changes in lung gene expression due to high tidal volume. Am J Respir Crit Care Med 168:1051–1059
Copland IB, Reynaud D, Pace-Asciak C, Post M (2006) Mechanotransduction of stretch-induced prostanoid release by fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 291:L487–L495
Copland IB, Post M (2007) Stretch-activated signaling pathways responsible for early response gene expression in fetal lung epithelial cells. J Cell Physiol 210:133–143
Garcia CS, Rocco PR, Fachinetti LD, Lassance RM, Caruso P, Deheinzelin D, Morales MM, Romero PV, Faffe DS, Zin WA (2004) What increases type III procollagen mRNA levels in lung tissue: stress induced by changes in force or amplitude? Respir Physiol Neurobiol 144:59–70
Breen EC (2000) Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro. J Appl Physiol 88:203–209
de Carvalho ME, Dolhnikoff M, Meireles SI, Reis LF, Martins MA, Deheinzelin D (2007) Effects of overinflation on procollagen type III expression in experimental acute lung injury. Critical Care 11:R23
Farias LL, Faffe DS, Xisto DG, Santana MC, Lassance R, Prota LF, Amato MB, Morales MM, Zin WA, Rocco PR (2005) Positive end-expiratory pressure prevents lung mechanical stress caused by recruitment/derecruitment. J Appl Physiol 98:53–61
Gutierrez JA, Perr HA (1999) Mechanical stretch modulates TGF-beta1 and alpha1(I) collagen expression in fetal human intestinal smooth muscle cells. Am J Physiol 277:G1074–1080
Schild C, Trueb B (2002) Mechanical stress is required for high-level expression of connective tissue growth factor. Exp Cell Res 274:83–91
Sheppard D (2006) Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc 3:413–417
Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D (2001) TGF-beta is a critical mediator of acute lung injury. J Clin Invest 107:1537–1544
Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, Matthay MA, Pittet JF (2003) Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 278:43939–43950
Deheinzelin D, Jatene FB, Saldiva PH, Brentani RR (1997) Upregulation of collagen messenger RNA expression occurs immediately after lung damage. Chest 112:1184–1188
Berg JT, Fu Z, Breen EC, Tran HC, Mathieu-Costello O, West JB (1997) High lung inflation increases mRNA levels of ECM components and growth factors in lung parenchyma. J Appl Physiol 83:120–128
Caruso P, Meireles SI, Reis LF, Mauad T, Martins MA, Deheinzelin D (2003) Low tidal volume ventilation induces proinflammatory and profibrogenic response in lungs of rats. Intensive Care Med 29:1808–1811
Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat J, Nicod LP, Chevrolet J (1998) Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol 275:L1040–1050
Foda HD, Rollo EE, Drews M, Conner C, Appelt K, Shalinsky DR, Zucker S (2001) Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340). Am J Respir Cell Mol Biol 25:717–724
Moriondo A, Pelosi P, Passi A, Viola M, Marcozzi C, Severgnini P, Ottani V, Quaranta M, Negrini D (2007) Proteoglycans fragmentation and respiratory mechanics in mechanically ventilated healthy rats. J Appl Physiol 103:747–756
Johnson Z, Proudfoot AE, Handel TM (2005) Interaction of chemokines and glycosaminoglycans: a new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev 16:625–636
Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM and Grone HJ (2005) The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115:2223–2233
Tran KT, Griffith L, Wells A (2004) Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen 12:262–268
Patel S, Santra M, McQuillan DJ, Iozzo RV, Thomas AP (1998) Decorin activates the epidermal growth factor receptor and elevates cytosolic Ca2+ in A431 carcinoma cells. J Biol Chem 273:3121–3124
Ludwig M (2007) Proteoglycans and pathophysiology. J Appl Physiol 103:735–736
D'Angelo E, Pecchiari M, Gentile G (2007) Dependence of lung injury on surface tension during low-volume ventilation in normal open-chest rabbits. J Appl Physiol 102:174–182
D'Angelo E, Pecchiari M, Della Valle P, Koutsoukou A, Milic-Emili J (2005) Effects of mechanical ventilation at low lung volume on respiratory mechanics and nitric oxide exhalation in normal rabbits. J Appl Physiol 99:433–444
D'Angelo E, Pecchiari M, Saetta M, Balestro E, Milic-Emili J (2004) Dependence of lung injury on inflation rate during low-volume ventilation in normal open-chest rabbits. J Appl Physiol 97:260–268
Milic-Emili J, Torchio R, D'Angelo E (2007) Closing volume: a reappraisal (1967–2007). Eur J Appl Physiol 99:567–583
Duggan M, McCaul CL, McNamara PJ, Engelberts D, Ackerley C, Kavanagh BP (2003) Atelectasis causes vascular leak and lethal right ventricular failure in uninjured rat lungs. Am J Respir Crit Care Med 167:1633–1640
Acknowledgements
We would like to express our gratitude to Prof. Rolf Hubmayr and Prof. Claudia Dos Santos for their suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-007-965-8.
Rights and permissions
About this article
Cite this article
Pelosi, P., Rocco, P.R. Effects of mechanical ventilation on the extracellular matrix. Intensive Care Med 34, 631–639 (2008). https://doi.org/10.1007/s00134-007-0964-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0964-9