Abstract
Background
Bronchoalveolar lavage fluid (BALF) is an important diagnostic source to investigate molecular changes occurring in lung disorders. The objective of this study was to assess and compare the peptidomic profiles of BALF from premature neonates with and without bronchopulmonary dysplasia (BPD).
Methods
Samples were obtained on the 3rd day of life from 34 neonates with gestational age ≤32 weeks. Two pools of samples from patients with and without BPD were analyzed by high performance liquid chromatography. Several differentially expressed peptides were collected and sequenced. Moreover, samples from single donors were analyzed by liquid chromatography-electrospray ionization mass spectrometry to define the molecular mass values of various peptides and to quantify their expression. Levels of some matrix metalloproteinases and their tissue inhibitors were also determined in single samples.
Results
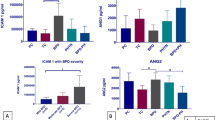
Neonates of the BPD group (N = 16) showed significantly lower mean gestational age and birth weight with respect to the no-BPD group (N = 18; P < 0.0001). Levels of six peptides were significantly higher in BPD patients (P < 0.05). Two of them were identified as the albumin fragments 1–21 (2,428 Da) and 399–406 (956 Da). Levels of matrix metalloproteinase-3 (MMP-3) enzyme probably involved in albumin fragment generation were also significantly higher in the BPD group compared to the no-BPD group (P < 0.05), whereas the levels of tissue inhibitor of metalloproteinases-1 were significantly lower (P < 0.05). Levels of albumin fragments and MMP-3 showed a significant correlation (P < 0.05).
Conclusions
This study shows that proteomic techniques can be applied to investigate the involvement of proteolytic enzymes on the airways of mechanically ventilated premature infants.




Similar content being viewed by others
References
Waittiez R, Falmagne P (2005) Proteomics of bronchoalveolar lavage fluid. J Chromatogr B Analyt Technol Biomed Life Sci 815:169–178
Bowler RP, Duda B, Chan ED, Enghild JJ, Ware LB, Matthay MA, Duncan MW (2004) Proteomic analysis of pulmonary edema fluid and plasma in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 286:L1095–L1104
Hirsch J, Hansen KC, Burlingame AL, Matthay MA (2004) Proteomics: current techniques and potential applications to lung disease. Am J Physiol Lung Cell Mol Physiol 287:L1–L23
Waittiez R, Hermans C, Bernard A, Lesur O, Falmagne P (1999) Human bronchoalveolar lavage fluid: two-dimensional gel electrophoresis, amino acid microsequencing and identification of major proteins. Electrophoresis 20:1634–1645
Waittiez R, Hermans C, Cruyt C, Bernard A, Falmagne P (2000) Human bronchoalveolar lavage fluid protein two dimensional database: study of interstitial lung diseases. Electrophoresis 21:2703–2712
Fietta AM, Bardoni AM, Salvini R, Passadore I, Morosini M, Cavagna L, Codullo V, Pozzi E, Meloni F, Montecucco C (2006) Analysis of bronchoalveolar lavage fluid proteome from systemic sclerosis patients with or without functional, clinical and radiological signs of lung fibrosis. Arthritis Res Ther 8:R160
Noël-Georis I, Bernard A, Falmagne P, Wattiez R (2001) Proteomics as the tool to search for lung disease markers in bronchoalveolar lavage. Dis Markers 17:271–284
Plymoth A, Löfdahl CG, Ekberg-Jansson A, Dahlbäck M, Broberg P, Foster M, Fehniger TE, Marko-Varga G (2007) Protein expression patterns associated with progression of chronic obstructive pulmonary disease in bronchoalveolar lavage of smokers. Clin Chem 53:636–644
Lenz AG, Meyer B, Costabel U, Maier K (1993) Bronchoalveolar lavage fluid proteins in human lung disease: analysis by two-dimensional electrophoresis. Electrophoresis 14:242–244
Lindahl M, Ståhlbom B, Tagesson C (1995) Two-dimensional gel electrophoresis of nasal and bronchoalveolar lavage fluids after occupational exposure. Electrophoresis 16:1199–1204
Lindahl M, Ståhlbom B, Tagesson C (1999) Newly identified proteins in human nasal and bronchoalveolar lavage fluids: potential biomedical and clinical applications. Electrophoresis 20:3670–3676
Magi B, Bini L, Perari MG, Fossi A, Sanchez JC, Hochstrasser D, Paesano S, Raggiaschi R, Santucci A, Pallini V, Rottoli P (2002) Bronchoalveolar lavage fluid protein composition in patients with sarcoidosis and idiopathic pulmonary fibrosis: a two-dimensional electrophoretic study. Electrophoresis 23:3434–3444
Sabounchi-Schütt F, Aström J, Hellman U, Eklund A, Grunewald J (2003) Changes in bronchoalveolar lavage fluid proteins in sarcoidosis: a proteomics approach. Eur Respir J 21:414–420
Ashton MR, Postle AD, Hall MA, Smith SL, Kelly FJ, Normand IC (1992) Phosphatidylcholine composition of endotracheal tube aspirates of neonates and subsequent respiratory disease. Arch Dis Child 67(4 Spec No):378–382
Vento G, Matassa PG, Ameglio F, Capoluongo E, Zecca E, Tortorolo L, Martelli M, Romagnoli C (2005) HFOV in premature neonates: effects on pulmonary mechanics and epithelial lining fluid cytokines. A randomized controlled trial. Intensive Care Med 31:463–470
Su BH, Watanabe T, Shimizu M, Yanagisawa M (1997) Echocardiographic assessment of patent ductus arteriosus shunt flow pattern in premature infants. Arch Dis Child Fetal Neonatal Ed 77:F36–F40
Bancalari E, Claure N (2006) Definitions and diagnostic criteria for bronchopulmonary dysplasia. Semin Perinatol 30:164–170
Vento G, Matassa PG, Zecca E, Tortorolo L, Martelli M, De Carolis MP, Maggio L, Zini G, D’Onofrio G, Valentini S, Romagnoli C (2004) Effect of dexamethasone on tracheobronchial aspirate fluid cytology and pulmonary mechanics in preterm infants. Pharmacology 71:113–119
Inzitari R, Vento G, Capoluongo E, Boccacci S, Fanali C, Cabras T, Romagnoli C, Giardina B, Messana I, Castagnola M (2007) Proteomic analysis of salivary acidic proline-rich proteins in human pre-term and at-term newborns. J Proteome Res 6:1371–1377
Ong SE, Mann M (2005) Mass spectrometry-based proteomics turns quantitative. Nat Chem Biol 1:252–262
Dargaville PA, South M, Vervaart P, McDougall PN (1999) Validity of markers of dilution in small volume lung lavage. Am J Respir Crit Care Med 160:778–784
Sellers A, Murphy G (1981) Collagenolytic enzymes and their naturally occurring inhibitors. Int Rev Connect Tissue Res 9:151–190
Nerusu KC, Warner RL, Bhagavathula N, McClintock SD, Johnson KJ, Varani J (2007) Matrix metalloproteinase-3 (stromelysin-1) in acute inflammatory tissue injury. Exp Mol Pathol 83:169–176
Fligiel SE, Standiford T, Fligiel HM, Tashkin D, Strieter RM, Warner RL, Johnson KJ, Varani J (2006) Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol 37:422–430
Warner RL, Beltran L, Younkin EM, Lewis CS, Weiss SJ, Varani J, Johnson KJ (2001) Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol 24:537–544
Wang M, Qin X, Mudgett JS, Ferguson TA, Senior RM, Welgus HG (1999) Matrix metalloproteinase deficiencies affect contact hypersensitivity: stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc Natl Acad Sci USA 96:6885–6889
Ekekezie II, Thibeault DW, Simon SD, Norberg M, Merrill JD, Ballard RA, Ballard PL, Truog WE (2004) Low levels of tissue inhibitors of matrix metalloproteinases with a high matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio are present in tracheal aspirate fluids of infants who develop CLD. Pediatrics 113:1709–1714
Tambunting F, Beharry KD, Hartleroad J, Waltzman J, Stavitsky Y, Modanlou HD (2005) Increased lung matrix metalloproteinase-9 levels in extremely premature baboons with bronchopulmonary dysplasia. Pediatr Pulmonol 39:5–14
Danan C, Jarreau PH, Franco ML, Dassieu G, Grillon C, Abd Alsamad I, Lafuma C, Harf A, Delacourt C (2002) Gelatinase activities in the airways of premature infants and development of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 283:L1086–L1093
Dik WA, van Kaam AH, Dekker T, Naber BA, Janssen DJ, Kroon AA, Zimmermann LJ, Versnel MA, Lutter R (2006) Early increased level of matrix metalloproteinase-9 in neonates recovering from respiratory distress syndrome. Biol Neonate 89:6–14
Sweet DG, Curley AE, Chesshyre E, Pizzotti J, Wilbourn MS, Halliday HL, Warner JA (2004) The role of matrix metalloproteinases-9 and -2 in development of neonatal chronic lung disease. Acta Paediatr 93:791–796
Cederqvist K, Sorsa T, Tervahartiala T, Maisi P, Reunanen K, Lassus P, Andersson S (2001) Matrix metalloproteinases-2, -8, and -9 and TIMP-2 in tracheal aspirates from preterm infants with respiratory distress. Pediatrics 108:686–692
Schulz CG, Sawicki G, Lemke RP, Roeten BM, Schulz R, Cheung PY (2004) MMP-2 and MMP-9 and their tissue inhibitors in the plasma of preterm and term neonates. Pediatr Res 55:794–801
Acknowledgments
The authors thank the Intensive Care Unit nursing staff for their invaluable collaboration on this work. They also are thankful for the financial support of Università Cattolica in Rome, MIUR, the Italian National Research Council (CNR), Università di Cagliari, Regione Sardegna and thanks fo the Fondazione Banco di Sardegna, International Scientific Institute “Paolo VI” (ISI) for their programs of scientific research promotion and diffusion. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vento, G., Tirone, C., Lulli, P. et al. Bronchoalveolar lavage fluid peptidomics suggests a possible matrix metalloproteinase-3 role in bronchopulmonary dysplasia. Intensive Care Med 35, 2115–2124 (2009). https://doi.org/10.1007/s00134-009-1646-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1646-6