Abstract
Summary
We evaluated the relation between serum FGF23 and bone mineral density (BMD) in a community-based cohort of elderly men. There was a weak correlation between FGF23 and BMD, which was primarily dependent on body weight.
Introduction
FGF23 is a hormonal factor produced in bone and regulates serum levels of phosphate (Pi) and vitamin D. FGF23 over-expression is associated with skeletal abnormalities, including rickets/osteomalacia. The relation between FGF23 and Bone Mineral Density (BMD) in the community remains unexplored.
Methods
We employed a large, population-based cohort of 3014 Swedish men aged 69–80 years, without known renal disease. BMD was measured with dual X-ray absorptiometry (DXA) in the hip and lumbar spine. Serum intact FGF23 was analyzed with a two-site monoclonal ELISA.
Results
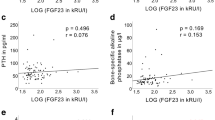
There was a weak but significant correlation between FGF23 and BMD in femoral neck (r = 0.04, p < 0.05), femoral trochanter (r = 0.05, p = 0.004), total hip (r = 0.06, p = 0.0015) and lumbar spine (r = 0.07, p = 0.0004). The correlations remained significant when adjusting for biochemical covariates (Pi, calcium, PTH, 25(OH)D and renal function). However, the association became insignificant in all regions when adjusting for established confounding variables including age, height, weight and smoking. Further analysis confirmed a significant correlation between FGF23 and body weight (r = 0.13, p < 0.0001).
Conclusions
The weak correlation between FGF23 and BMD in elderly male subjects is mainly due to an association between FGF23 and body weight. Therefore, FGF23 may not play a significant role in the hormonal regulation of BMD.


Similar content being viewed by others
References
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98(11):6500–6505
Imel EA, Econs MJ (2007) Fibrous dysplasia, phosphate wasting and fibroblast growth factor 23. Pediatr Endocrinol Rev 4 Suppl 4:434–439
Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, White KE (2005) A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab 90(4):2424–2427
Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB (2003) Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64(6):2272–2279
Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT (2004) FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab 89(9):4489–4492
Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P (2003) FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112(5):683–692
Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD (2003) Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem 278(39):37419–37426
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113(4):561–568
Ito M, Sakai Y, Furumoto M, Segawa H, Haito S, Yamanaka S, Nakamura R, Kuwahata M, Miyamoto K (2005) Vitamin D and phosphate regulate fibroblast growth factor-23 in K-562 cells. Am J Physiol Endocrinol Metab 288(6):E1101–E1109
Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB (2004) Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145(7):3087–3094
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444(7120):770–774
Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, Aubin JE, Maeda N (2008) Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res 23(6):939–948 Jun
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390(6655):45–51
White KE, Larsson TM, Econs MJ (2006) The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: Frp-4, MEPE, and FGF23. Endocr Rev 27(3):221–241
Mellstrom D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H, Holmberg A, Redlund-Johnell I, Orwoll E, Ohlsson C (2006) Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 21(4):529–535
Lu Y, Fuerst T, Hui S, Genant HK (2001) Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int 12(6):438–444
Genant HK, Grampp S, Gluer CC, Faulkner KG, Jergas M, Engelke K, Hagiwara S, Van Kuijk C (1994) Universal standardization for dual X-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res 9(10):1503–1514
Hanson J (1997) Standardization of proximal femur BMD measurements. International Committee for Standards in Bone Measurement. Osteoporos Int 7(5):500–501
Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S (2002) Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87(11):4957–4960
Marsell R, Grundberg E, Krajisnik T, Mallmin H, Karlsson M, Mellstrom D, Orwoll E, Ohlsson C, Jonsson KB, Ljunggren O, Larsson TE (2008) Fibroblast growth factor-23 is associated with parathyroid hormone and renal function in a population-based cohort of elderly men. Eur J Endocrinol 158(1):125–129
Flodin M, Jonsson AS, Hansson LO, Danielsson LA, Larsson A (2007) Evaluation of Gentian cystatin C reagent on Abbott Ci8200 and calculation of glomerular filtration rate expressed in mL/min/1.73 m(2) from the cystatin C values in mg/L. Scand J Clin Lab Invest 67(5):560–567
Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H (2003) Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348(17):1656–1663
Urena Torres P, Friedlander G, de Vernejoul MC, Silve C, Prie D (2008) Bone mass does not correlate with the serum fibroblast growth factor 23 in hemodialysis patients. Kidney Int 73(1):102–107
Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G (2006) Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest 116(12):3150–3159 Dec
Acknowledgements
We are in sincere debt and are deeply grateful to the senior professor Olof Johnell, who played a vital role during the initiation phase of the MrOS study.
The authors would also like to thank Anna-Lena Johansson, Violja Mixhue, Karin Önnby, Inger Abrahamsson, Maud Petterson, Ann-Charlotte Adolfsson and Marja Gustafsson for excellent technical assistance.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Richard Marsell and Majd A. I. Mirza contributed equally to this work.
Funding source: this study was supported by the Swedish Research Council, the Novo Nordisk Foundation, the Swedish Kidney Foundation and the Swedish Society of Medicine.
Rights and permissions
About this article
Cite this article
Marsell, R., Mirza, M.A.I., Mallmin, H. et al. Relation between fibroblast growth factor-23, body weight and bone mineral density in elderly men. Osteoporos Int 20, 1167–1173 (2009). https://doi.org/10.1007/s00198-008-0780-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0780-2