Abstract
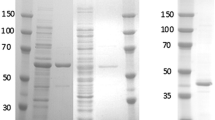
Clostridium aminovalericum, an obligate anaerobe, is unable to form colonies on PYD agar plates in the presence of 1% O2. When grown anaerobically in PYD liquid medium, the strain can continue normal growth after the shift from anoxic (sparged with O2-free N2 carrier-gas) to microoxic (sparged with 3% O2/97% N2 mixed carrier-gas) growth conditions in the mid exponential phase (OD660=1.0). When the strain grew under 3% O2/97% N2, the medium remains anoxic. Thirty minutes after beginning aeration with 3% O2, the activity of NADH oxidase in cell-free extracts increased more than five-fold from the level before aeration. We purified NADH oxidase to determine the characteristics of this enzyme in an obligate anaerobe. The purified NADH oxidase dominated the NADH oxidase activity detected in cell-free extracts. The enzyme is a homotetramer composed of a subunit with a molecular mass of 45 kDa. The enzyme shows a spectrum typical of a flavoprotein, and flavin adenine dinucleotide (FAD) was identified as a cofactor. The final product of NADH oxidation was H2O, and the estimated K m for oxygen was 61.9 μM. These data demonstrate that an O2-response enzyme that is capable of detoxifying oxygen to water exists in C. aminovalericum.




Similar content being viewed by others
Abbreviations
- NRIC:
-
NODAI Research Institute-Culture Collection Center, Tokyo University of Agriculture, Tokyo, Japan
- SDS-PAGE:
-
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- PMSF:
-
phenylmethylsulfonyl fluoride
References
Andreesen JR, Bahl H, Gottschalk G (1991) Introduction to the physiology and biochemistry of the genus Clostridium. In: Minton NP, Clark DJ (eds) Biotechnology handbook, vol 3. Plenum Press, New York, pp 27–62
Appleby CA (1969) Inhibitors of respiratory enzymes, photosynthesis and phosphorylation; uncoupling reagents. In: Dawson RM, Elliott DC, Elliott WH, Jones KM (eds) Data for biochemical research. Oxford University Press, Oxford, pp 380–387
Auzat I, Chapuy-Regaud S, Bras GL, Santos DD, Ogunniyi AD, Thomas I L, Garel JR, Paton JC Trombe MC (1999) The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol Microbiol 34:1018–1028
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Contreras ML, Escamilla JE, Del Arenal IP, Davila JR, D’mello R, Poole RK (1999) An unusual cytochrome o’-type cytochrome c oxidase in a Bacillus cereus cytochrome a 3 mutant has a very high affinity for oxygen. Microbiology 145:1563–1573
D’Mello R, Hill S, Poole RK (1995) The oxygen affinity of cytochrome bo’ in Escherichia coli determined by the deoxygenation of oxyleghemoglobin and oxymyoglobin: K m values for oxygen are in the submicromolar range. J Bacteriol 177:867–870
Gibson CM, Mallett TC, Claiborne A, Caparon MG (2000) Contribution of NADH oxidase to aerobic metabolism of Streptococcus pyogenes. J Bacteriol 182:448–455
Herles C, Braune A, Blaut M 2002. Purification and characterization of an NADH oxidase from Eubacterium ramulus. Arch Microbiol 178:71–74
Higuchi M, Yamamoto Y, Poole LB, Shimada M, Sato Y, Takahashi N, Kamio Y (1999) Functions of two types of NADH oxidases in energy metabolism and oxidative stress of Streptococcus mutans. J Bacteriol 181:5940–5947
Holdeman LV, Cato EP, Moor WEC (1997) Anaerobe laboratory manual, 4th edn. Anaerobe Laboratory, Virginia Polytechnic Institute and State University
Holland KT, Knapp JS, Shoesmith JG (1987) Anaerobes and oxygen. In: Holland KT, Knapp JS, Shoesmith JG (eds) Anaerobic bacteria. Chapman and Hall, New York, pp 4–12
Kawasaki S, Nakagawa T, Nishiyama Y, Benno Y, Uchimura T, Komagata K, Kozaki K, Niimura Y (1998) Effect of oxygen on the growth of Clostridium butyricum (type species of the genus Clostridium), and the distribution of enzymes for oxygen and for active oxygen species in Clostridia. J Ferment Bioeng 86:368–372
Logan C, Mayhew SG (2000) Cloning, overexpression, and characterization of peroxiredoxin and NADH peroxiredoxin reductase from Thermus aquaticus. J Biol Chem 275:30019–30028
Maeda K, Truscott K, Liu XL, Scopes K (1992) A thermostable NADH oxidase from anaerobic extreme thermophiles. Biochem J 284:551–555
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Mitchell WJ (2001) Biology and physiology. In: Bahl H, Dürre P (eds) Clostridia. Wiley–VCH, Weinheim, Germany, pp 49–104
Morris JG (1976) Oxygen and the obligate anaerobe. J Appl Bacteriol 40:229–244
van Niel EWJ, Hofvendahl K, Hahn-Hägerdal B (2002) Formation and conversion of oxygen metabolites by Lactococcus lactis subsp. lactis ATCC 19435 under different growth conditions. Appl Environ Microbiol 68:4350–4356
Niimura Y, Kho E, Uchimura T, Ohara N, Kozaki K (1989) Aerobic and anaerobic metabolism in a facultative anaerobe EP01 lacking cytochrome, quinone, and catalase. FEMS Microbiol Lett 61:79–84
Niimura Y, Kho E, Yanagida F, Suzuki K, Komagata K, Kozaki M (1990) Amphibacillus xylanus gen. nov., sp. nov., a facultatively anaerobic sporeforming xylan-digesting bacterium which lacks cytochrome, quinone, and catalase. Int J Syst Bacteriol 40:297–301
Niimura Y, Poole LB, Massey V (1995) Amphibacillus xylanus NADH oxidase and Salmonella typhimurium alkyl-hydroperoxide reductase flavoprotein components show extremely high scavenging activity for both alkyl hydroperoxide and hydrogen peroxide in the presence of S. typhimurium alkyl-hydroperoxide reductase 22-kDa protein component. J Biol Chem 270:25645–25650
Niimura Y, Ohnishi K, Nishiyama Y, Kawasaki S, Miyaji T, Suzuki H, Nishino T, Massey V (1997) Amphibacillus xylanus NADH oxidase/alkyl hydroperoxide reductase flavoprotein. In: Stevenson KJ, Massey V, Williams CH (eds) Flavins and flavoproteins 1996. University of Calgary Press, Calgary, Canada, pp 741–750
Niimura Y, Nishiyama Y, Saito D, Tsuji H, Hidaka M, Miyaji T, Watanabe T, Massey V (2000) A hydrogen peroxide-forming NADH oxidase that functions as an alkyl hydroperoxide reductase in Amphibacillus xylanus. J Bacteriol 182:5046–5051
Nishiyama Y, Massey V, Anzai Y, Watanabe T, Miyaji T, Uchimura T, Kozaki M, Suzuki H, Niimura Y (1997) Purification and characterization of Sporolactobacillus inulinus NADH oxidase and its physiological role in aerobic metabolism of the bacterium. J Ferment Bioeng 84:22–27
O’Brien RW, Morris JG (1971) Oxygen and growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol 68:307–318
Ohnishi K, Niimura Y, Yokoyama K, Hidaka M, Masaki H, Uchimura T, Suzuki H, Uozumi T, Kozaki M, Komagata K, Nishino T (1994) Purification and analysis of a flavoprotein functional as NADH oxidase from Amphibacillus xylanus overexpressed in Escherichia coli. J Biol Chem 269:31418–31423
Poole LB, Reynolds CM, Wood ZA, Karplus PA, Ellis HR, Calzi LM (2000) AhpF and other NADH:peroxiredoxin oxidoreductases, homologues of low Mr thioredoxin reductase. Eur J Biochem 267:6126–6133
Preisig O, Zufferey R, Thony-Meyer L, Appleby CA, Hennecke H. (1996) A high-affinity cbb 3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J Bacteriol 178:1532–1538
Ross RP, Claiborne A (1992) Molecular cloning and analysis of the gene encoding the NADH oxidase from Streptococcus faecalis 10C1. J Mol Biol 227:658–671
Seaver LC, Imlay JA (2001) Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol 183:7173–7181
Sneath PHA (1986) Endospore-forming gram-positive rods and cocci. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 2. Williams and Wilkins Co., Baltimore, pp 1104–1207
Stanton TB, Rosey EL, Kennedy MJ, Jensen NS, Bosworth BT (1999) Isolation, oxygen sensitivity, and virulence of NADH oxidase mutants of the anaerobic spirochete Brachyspira (Serpulina) hyodysenteriae, etiologic agent of swine dysentery. Appl Environ Microbiol 65:5028–5034
Storz G, Tartaglia LA, Farr SB, Ames BN (1990) Bacterial defenses against oxidative stress. Trends Genet 6:363–368
Trudinger PA (1969) Thiol reagents. In: Dawson RM, Elliott DC, Elliott WH, Jones KM (eds) Data for biochemical research. Oxford University Press, Oxford, pp 388–395
Ward DE, Donnelly CJ, Mullendore ME, van der Oost J, de Vos WM, Crane EJ III (2001) The NADH oxidase from Pyrococcus furiosus. Implications for the protection of anaerobic hyperthermophiles against oxidative stress. Eur J Biochem 268:5816–5823
Woods DR, Jones DT (1986) Physiological responses of Bacteroides and Clostridium strains to environmental stress factors. Adv Microb Physiol 28:1–64
Yu J, Bryant AP, Marra A, Lonetto MA, Ingraham KA, Chalker AF, Holmes DJ, Holden D, Rosenberg M, McDevitt D (2001) Characterization of the Streptococcus pneumoniae NADH oxidase that is required for infection. Microbiology 147:431–438
Acknowledgments
We thank Dr Takeshi Nishino, Dr Kenji Ohnishi, and Dr Tomoyuki Nakagawa for helpful suggestion and valuable discussion, and Mr Yusuke Watamura and Mr Satoshi Nakayama, Mr Masaki Ono, Mr Tetsuya Matsumoto, and Mr Tatsuya Noguchi for helpful technical assistance at Tokyo University of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawasaki, S., Ishikura, J., Chiba, D. et al. Purification and characterization of an H2O-forming NADH oxidase from Clostridium aminovalericum: existence of an oxygen-detoxifying enzyme in an obligate anaerobic bacteria. Arch Microbiol 181, 324–330 (2004). https://doi.org/10.1007/s00203-004-0659-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-004-0659-3