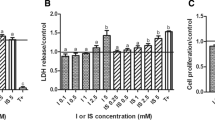
Abstract
The metabolic competence of cultured bovine colon epithelial cells was evaluated by determining activities of phase I and II enzymes in colonocytes cultured for different intervals (maximum of 10 days) compared with activities measured in freshly isolated cells. Cytochrome P450 1A1-associated 7-ethoxyresorufin O-deethylase (EROD) activity was detectable in freshly isolated colonocytes and in colon cells maintained in culture for up to 5 days. In contrast to liver samples, cytochrome P450 3A4-associated 7-benzyloxyresorufin O-debenzylase (BROD) activity was not detectable in bovine colon cells. Prostaglandin H synthase-mediated production of prostaglandin E2 was found in freshly isolated and also in cultured colonocytes. Both isoenzymes (COX 1 and COX 2) were detected in cultured cells. To examine phase II metabolic potency, activities of N-acetyltransferases 1 and 2, of phenol and amino sulfotransferases, of glutathione S-transferases alpha, mu, pi and theta and of UDP-glucuronyltransferase were measured. N-Acetyltransferase (NAT) activity (substrate p-aminobenzoic acid, PABA, a diagnostic substrate for the human NAT-1 enzyme) was stable under culture conditions and during the observed culture period comparable to that of freshly isolated cells. In contrast, sulfamethazine, a specific substrate for NAT-2, was not acetylated, neither in bovine colon cells nor in bovine liver samples. Whereas activity of amino sulfotransferase (substrate 2-naphthylamine) decreased continuously during the entire culture period, the activity of phenol sulfotransferase (substrate 1-naphthol) decreased only slowly. Activity of total glutathione S-transferases (alpha, mu, and pi) (substrate 1-chloro-2,4-dinitrobenzene) decreased after 2 days in culture, but was stable during the following culture period. Activity of glutathione S-transferase theta (substrate epoxy-3-nitrophenoxypropane) changed during the culture period. At the beginning and the end (after 10 days) of the culture period maximum activity was measured. Activity of UDP-glucuronyltransferase increased during the culture period reaching a maximum after 7 days. The results show that cultured bovine epithelial colon cells express several enzyme activities required for the biotransformation of xenobiotics.







Similar content being viewed by others
References
Bartoli R, Fernandez-Banares F, Navarro E, Castella E, Mane J, Alvarez M, Pastor C, Cabre E, Gassull MA (2000) Effect of olive oil on early and late events of colon carcinogenesis in rats: modulation of arachidonic acid metabolism and local prostaglandin E2 synthesis. Gut 46:191–199
Baten A, Sakamoto K, Shamsuddin AM (1992) Long-term culture of normal human colonic epithelial cells in vitro. FASEB J 6:2726–2734
Booth C, Patel S, Bennion GR, Potten CS (1995) The isolation and culture of adult mouse colonic epithelium. Epithelial Cell Biol 4:76–86
Burke MD, Meyer RT (1974) Ethoxyresorufin: direct fluorimetric assay of a microsomal O-dealkylation which is preferentially inducible by 3-methylcholanthene. Drug Metab Dispos 2:583–588
Butler RN, Butler WJ, Moraby Z, Fettman MJ, Khoo KK, Roberts-Thomson IC (1994) Glutathione concentrations and glutathione S-transferase activity in human colonic neoplasms. J Gastroenterol Hepatol 9:60–63
Cappiello M, Giuliani L, Pacifici GM (1990) Differential distribution of phenol and catechol sulphotransferases in human liver and intestinal mucosa. Pharmacology 40:69–76
Carriere V, Lesuffleur T, Barbat A, Rousset M, Dussaulx E, Costet P, de Waziers I, Beaune P, Zweibaum A (1994) Expression of cytochrome P-450 3A in HT 29-MTX cells and Caco-2 clone TC7. FEBS Lett 355:247–250
Davis CD, Schut HAJ, Snyderwine EG (1993) Enzymatic phase II activation of the N-hydroxylamines of IQ, MelQx and PhiP by various organs of monkeys and rats. Carcinogenesis 14:2091–2096
de Waziers I, Cugnenc PH, Yang CS, Leroux JP, Beaune PH (1990) Cytochrome P450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J Pharmacol Exp Ther 253:387–393
Deveney CW, Rand-Luby L, Rutten MJ, Luttropp CA, Fowler WM, Land J, Meichsner CL, Farahmand M, Sheppard BC, Crass RA, Deveney MD (1996) Establishment of human colonic epithelial cells in long-term culture. J Surg Res 64:161–169
Donato MT, Gomez-Lechon MJ, Castell JV (1990) Drug metabolizing enzymes in rat hepatocytes co-cultured with cell lines. In Vitro Cell Dev Biol 26:1057–1062
Dutton DR, Parkinson A (1989) Reduction of 7-alkoxyresorufins by NADPH-cytochrome P450 reductase and its differential effects on their O-dealkylation by rat liver microsomal cytochrome P450. Arch Biochem Biophys 268; 617–629
Eling TE, Thompson DC, Foureman GL, Curtis JF, Hughes MF (1990) Prostaglandin H synthase and xenobiotic oxidation. Annu Rev Pharmacol Toxicol 30:1–45
Föllmann W, Weber S, Birkner S (2000) Primary cell cultures of bovine colon epithelium: isolation and cell culture of colonocytes. Toxicol In Vitro 14:435–445
Fonti R, Latella G, Bises G, Magliocca F, Nobili F, Caprilli R, Sambuy Y (1994) Human colonocytes in primary culture: a model to study epithelial growth, metabolism and differentiation. Int J Colorect Dis 9:13–22
Freshney RJ (ed) (1987) Culture of animal cells. Alan R Liss, New York
Guhe C, Degen GH, Schuhmacher US, Kiefer F, Föllmann W (1996) Drug metabolizing enzyme activities in porcine urinary bladder epithelial cell cultures (PUBEC). Arch Toxicol 70:599–606
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. J Biol Chem 249:7130–7139
Halliman T (1984) UDP-glucuronosyltransferases. In: Bergmeyer H (ed) Methods of enzymatic analysis. Verlag Chemie, Weinheim
Hänninen O, Lindsrröm-Seppä P, Pelkonen K (1987) Role of gut in xenobiotic metabolism. Arch Toxicol 60:34–36
Hata Y, Ota S, Nagata T, Uehara Y, Terano A, Sugimoto T (1993) Primary colonic epithelial cell culture of the rabbit producing prostaglandins. Prostaglandins 45:129–141
Howie AF, Forrester LM, Glancey MJ, Schlager JJ, Powis G, Beckett GJ, Hayes JD, Wolf CR (1990) Glutathione S-transferase and glutathione peroxidase expression in normal and tumour human tissue. Carcinogenesis 11:451–458
Illet KF, Reeves PT, Minchin RF, Kinnear BF, Watson HF, Kadbular FF (1991) Distribution of acetyltransferase activities in the intestines of rapid and slow acetylator rabbits. Carcinogenesis 12:1465–1469
Kamitani H, Geller M, Eling T (1998) Expression of 15-lipoxygenase by human colorectal carcinoma Caco-2 cells during apoptosis and cell differentiation. J Biol Chem 273:21569–21577
Kawakubo Y, Manabe S, Yamazoe Y, Nishikawa T, Kato R (1988) Properties of cutaneous acetyltransferase catalyzing N- and O-acetylation of carcinogenic arylamines and N-hydroxyarylamine. Biochem Pharmacol 37:265–270
Kenworthy KE, Bloomer JC, Clarke SE, Houston JB (1999) CYP 3A4 drug interactions: correlation of 10 in vitro probe substrates. Br J Clin Pharmacol 48:716–727
Massaad L, de Waziers I, Ribrag V, Janot F, Beaune PH, Morizet J, Gouyette A, Chabot GG (1992) Comparison of mouse and human colon tumors with regard to phase I and phase II drug-metabolizing enzyme system. Cancer Res 52:6567–6575
Matsubara T, Koike M, Touchi A, Tochino Y, Sugeno K (1976) Quantitative determination of cytochrome P-450 in rat liver homogenate. Anal Biochem 75:96–99
Nygard G, Larsson A, Gerdin B, Ejerblad S, Berglindh T (1992) Viability, prostaglandin E2 production and protein handling in normal and inflamed human colonic mucosa cultured for up to 48 h in vitro. Scand J Gastroenterol 27:303–310
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. J Biol Chem 239:2370–2378
Pacifici GM, Bencini C, Rane A (1986) Acetyltransferase in humans: development and tissue distribution. Pharmacology 32:283–291
Peters WH, Roelofs HM (1989) Time-dependent activity and expression of glutathione S-transferase in human colon adenocarcinoma cell line Caco-2. Biochem J 264:613–616
Peters WHM, Kock FM, Nagengast FM, Kremers PK (1991) Biotransformation enzymes in human intestine: critical low levels in the colon? Gut 32:408–412
Petry TW, Josephy PD, Pagano DA, Zeiger E, Knecht KT, Eling TE (1989) Prostaglandin hydroperoxidase-dependent activation of heterocyclic aromatic amines. Carcinogenesis 10:2201–2207
Pool-Zobel BL, Leucht U (1997) Induction of DNA damage by risk factors of colon cancer in human colon cells derived from biopsies. Mutat Res 375:105–115
Raeissi SD, Guo Z, Dobson GL, Artursson P, Hidalgo IJ (1997) Comparison of CYP3A in subclone of Caco-2 cells (TC7) and human intestine. Pharmacol Res 14:1019–1025
Ramaswamy SG Jacoby WB (1987) Sulfotransferase assays. Methods Enzymol 143:201–207
Rosenberg DW, Lefft T (1993) Regulation of cytochrome P450 in cultured human colonic cells. Arch Biochem Biophys 300:186–192
Sasaki YF, Saga A, Yoshida K, Su Y Q, Ohta T, Matsusaka N, Tsuda S (1998) Colon-specific genotoxicity of heterocyclic amines detected by the modified alkaline single cell gel electrophoresis assay of multiple mouse organs. Mutat Res 414:9–14
Schut HAJ, Snyderwine EG (1999) DNA adducts of heterocyclic amine food mutagens: implication for mutagenesis and carcinogenesis. Carcinogenesis 20:353–368
Schwenk M (1988) Mucosal biotransformation. Toxicol Pathol 16:138–146
Shimada T, Yun CH, Yamazaki H, Gautier J-C, Beaune PH, Guengerich FP (1992) Characterization of human microsomal cytochrome P 450 1A1 and its role in the oxidation of chemical carcinogens. Mol Pharmacol 41:856–864
Styczynski PB, Blackman RC, Groopman JD, Kensler TW (1993) The direct glucuronidation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhiP) by human and rabbit liver microsomes. Chem Res Toxicol 6:846–851
Thompson AA, Dilworth S, Hay RJ (1985) Isolation and culture of colonic epithelial cells in serum-free medium. J Tissue Culture Methods 9:117–122
Turesky RJ, Lang NP, Butler MA, Teitel CH, Kadlubar FF (1991) Metabolic activation of carcinogenic heterocyclic aromatic amines by human liver and colon. Carcinogenesis 12:1839–1845
Vainio H (1980) Role of hepatic metabolism. In: Testa B, Jenner P (eds) Concepts in drug metabolism. Dekker, New York, pp 251–284
van't Klooster GAE, Woutersen-van Nijnanten FMA, Blaauboer BJ, Noordhoek J, van Miert A (1994) Application of cultured hepatocytes from goat, sheep and cattle in comparative drug metabolism studies. Xenobiotica 24:417–428
Wild D, Degen GH (1987) Prostaglandin H synthase-dependent mutagenic activation of heterocyclic aromatic amine of the IQ type. Carcinogenesis 8:541–545
Wolz E, Wild D, Degen GH (1995) Prostaglandin H synthase mediated metabolism and mutagenic activation of 2-amino-3-methylimidazo[4,5-f]-quinoline (IQ). Arch Toxicol 69:171–179
Yeh K-Y, Chorpra DP (1980) Epithelial cell cultures from the colon of the suckling rat. In Vitro 16:976–986
Acknowledgements
The authors gratefully acknowledge the excellent technical assistance of B. Kullik, M. Koch, A. Dommermuth and C. Pütt. Special thanks are due to Professor Dr. F. Wiebel (GSF Neuherberg, Germany) for cooperation that allowed us to perform the NAT measurements, and to Dr. C. Vogel (Medizinisches Institut für Umwelthygiene, Düsseldorf) for the support with the PCR methods. This work was supported by grant 0311266 from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Birkner, S., Weber, S., Dohle, A. et al. Activities of drug metabolizing enzymes in bovine colon epithelial cell cultures. Arch Toxicol 77, 621–629 (2003). https://doi.org/10.1007/s00204-003-0490-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0490-7