Abstract
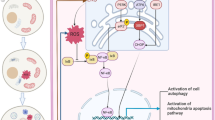
Oxidative stress induces activation of extracellular signal-regulated kinase (ERK), a member of the mitogen-activated protein kinase families. However, it is unclear in renal epithelial cells whether the ERK activation is involved in cell survival or cell death in H2O2-treated cells. The present study was undertaken to determine the role of the ERK activation in H2O2-induced apoptosis of renal epithelial cells using opossum kidney (OK) cells, an established proximal tubular epithelial cell line. H2O2 resulted in a time- and dose-dependent apoptosis of OK cells. H2O2 treatment caused marked sustained activation of ERK. The ERK activation was prevented by PD98059 and U0126, inhibitors of ERK1/2 upstream kinase MEK1/2. Apoptosis caused by H2O2 was prevented by U0126. Transient transfection with constitutive active MEK1 increased the H2O2-induced apoptosis, whereas transfection with dominant-negative mutants of MEK1 decreased the apoptosis. H2O2 produced hyperpolarization of mitochondrial membrane potential and activation of caspases-3. H2O2-induced ERK activation was inhibited by the Src family selective inhibitor PP2 and the epidermal growth factor receptor inhibitor AG1478. The presence of AG1478, but not PP2, prevented H2O2-induced cell death. Taken together, our findings suggest that the ERK activation mediated by epidermal growth factor receptor plays an active role in inducing H2O2-induced apoptosis of OK cells and functions upstream of mitochondria-dependent pathway to initiate the apoptotic signal.






Similar content being viewed by others
References
Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL (2004) Activation of ERK or inhibition of JNK ameliorates H2O2 cytotoxicity in mouse renal proximal tubule cells. Kidney Int 65:1231–1239
Baek SM, Kwon CH, Kim JH, Woo JS, Jung JS, Kim YK (2003) Differential roles of hydrogen peroxide and hydroxyl radical in cisplatin-induced cell death in renal proximal tubular epithelial cells. J Lab Clin Med 142:178–186
Banki K, Hutter E, Gonchoroff NJ, Perl A (1999) Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J Immunol 162:1466–1479
Baud L, Ardaillou R (1986) Reactive oxygen species: production and role in the kidney. Am J Physiol 251:F765–F776
Bhat NR, Zhang P (1999) Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line: role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J Neurochem 72:112–119
Brand A, Gil S, Seger R, Yavin E (2001) Lipid constituents in oligodendroglial cells alter susceptibility to H2O2-induced apoptotic cell death via ERK activation. J Neurochem 76:910–918
Chen M, Jahnukainen T, Bao W, Dare E, Ceccatelli S, Celsi G (2003) Uropathogenic Escherichia coli toxins induce caspase-independent apoptosis in renal proximal tubular cells via ERK signaling. Am J Nephrol 23:140
Cobb MH (1999) MAP kinase pathways. Prog Biophys Mol Biol 71:479–500
Cuttle L, Zhang X-J, Endre ZH, Winterford C, Gobe GC (2001) Bcl-XL translocation in renal tubular epithelial cells in vitro protects distal cells from oxidative stress. Kidney Int 59:1779–1788
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252
di Mari JF, Davis R, Safirstein RL (1999) MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol 277:F195–F203
Diamond JR, Bonventre JV, Karnovsky MJ (1986) A role for oxygen free radicals in aminonucleoside nephrosis. Kidney Int 29:478–483
Dong J, Ramachandiran S, Tikoo K, Jia Z, Lau SS, Monks TJ (2004) EGFR-independent activation of p38 MAPK and EGFR-dependent activation of ERK1/2 are required for ROS-induced renal cell death. Am J Physiol 287:F1049–F1058
Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR (1995) A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA 92:7686–7689
Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273:18623–18632
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A (2001) Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for inter receptor signal transmission. Oncogene 20:1594–1600
Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ (1996) Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem 271:4138–4142
Ishikawa Y, Kitamura M (2000) Anti-apoptotic effect of quercetin: intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int 58:1078–1087
Kim YK, Kim HJ, Kwon CH, Kim JH, Woo JS, Jung Js, Kim JM (2005) Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J Appl Toxicol 25:374–382
Kroemer G, Dallaporta B, Resche-Rigon M (1998) The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol 60:619–642
Lemasters JJ, Nieminen AL, Qian T, Trost LC, Herman B (1997) The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol Cell Biochem 174:159–165
Leonard SS, Harris GK, Shi X (2004) Metal-induced oxidative stress and signal transduction. Free Radic Biol Med 37:1921–1942
Lieberthal W, Levine JS (1996) Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol 271:F477–F488
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
Meves A, Stock SN, Beyerle A, Pittelkow MR, Peus D (2001) H(2)O(2) mediates oxidative stress-induced epidermal growth factor receptor phosphorylation. Toxicol Lett 122:205–214
Nowak G (2002) PKC-a and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J Biol Chem 277:43377–43388
Paller MS, Hoidal JR, Ferris TF (1984) Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest 74:1156–1164
Pastorino JG, Snyder JW, Serroni A, Hoek JB, Farber JL (1993) Cyclosporin and carnitine prevent the anoxic death of cultured hepatocytes by inhibiting the mitochondrial permeability transition. J Biol Chem 268:13791–13798
Peus D, Meves A, Vasa RA, Beyerle A, O’Brien T, Pittelkow MR (1999) H2O2 is required for UVB-induced EGF receptor and downstream signaling pathway activation. Free Radic Biol Med 27:1197–1202
Piret JP, Arnould T, Fuks B, Chatelain P, Remacle J, Michiels C (2004) Mitochondria permeability transition-dependent tert-butyl hydroperoxide-induced apoptosis in hepatoma HepG2 cells. Biochem Pharmacol 67:611–620
Poppe M, Reimertz C, Dussmann H, Krohn AJ, Luetjens CM, Bockelmann D, Nieminen AL, Kogel D, Prehn JH (2001) Dissipation of potassium and proton gradients inhibits mitochondrial hyperpolarization and cytochrome c release during neural apoptosis. J Neurosci 21:4551–4563
Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A (2001) The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer 8:11–31
Puskas F, Gergely P Jr, Banki K, Perl A (2000) Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. Faseb J 14:1352–1361
Ramachandiran S, Huang O, Dong J, Lau SS, Monks TJ (2002) Mitogen-activated protein kinases contribute to reactive oxygen species-induced cell death in renal proximal tubular epithelial cells. Chem Res Toxicol 15:1635–1642
Rana A, Sathyanarayana P, Lieberthal W (2001) Role of apoptosis of renal tubular cells in acute renal failure: therapeutic implications. Apoptosis 6:83–102
Rehan A, Johnson KJ, Wiggins RC, Kunkel RG, Ward PA (1984) Evidence for the role of oxygen radicals in acute nephrotoxic nephritis. Lab Invest 51:396–403
Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf HJ (1994) Involvement of growth factor receptors in the mammalian UVC response. Cell 78:963–972
Sato K, Sato A, Aoto M, Fukami Y (1995) c-Src phosphorylates epidermal growth factor receptor on tyrosine 845. Biochem Biophys Res Commun 215:1078–1087
Takeda M, Shirato I, Kobayashi M, Endou H (1999) Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron 81:234–238
Tatton WG, Olanow CW (1999) Apoptosis in neurodegenerative diseases: the role of mitochondria. Biochim Biophys Acta 1410:195–213
Tian W, Zhang Z, Cohen DM (2000) MAPK signaling and the kidney. Am J Physiol Renal Physiol 279:F593–F604
Ueda N, Kaushal GP, Shah SV (2000) Apoptotic mechanisms in acute renal failure. Am J Med 108:403–415
Walker PD, Shah SV (1988) Evidence suggesting a role for hydroxyl radical in gentamicin-induced acute renal failure in rats. J Clin Invest 81:334–341
Wang X, Martindale JL, Liu Y, Holbrook NJ (1998) The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J 333:291–300
Waterhouse NJ, Goldstein JC, von Ahsen O, Schuler M, Newmeyer DD, Green DR (2001) Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J Cell Biol 153:319–328
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331
Zhougang S, Schnellmann RG (2004) H2O2-induced transactivation of EGF receptor requires Src and mediates ERK1/2, but not Akt, activation in renal cells. Am J Physiol Renal Physiol 286:F858–F865
Acknowledgement
This work was supported by grant R01-2002-000-00460-0 (2002) from the Basic Research Program of the Korean Science & Engineering foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.S., Kim, S.Y., Kwon, C.H. et al. EGFR-dependent ERK activation triggers hydrogen peroxide-induced apoptosis in OK renal epithelial cells. Arch Toxicol 80, 337–346 (2006). https://doi.org/10.1007/s00204-005-0052-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-005-0052-2