Abstract
Rationale
Previous studies have shown that orexin-1/hypocretin-1 receptors play a role in self-administration and cue-induced reinstatement of food, drug, and ethanol seeking. In the current study, we examined the role of orexin-1/hypocretin-1 receptors in operant self-administration of ethanol and sucrose and in yohimbine-induced reinstatement of ethanol and sucrose seeking.
Materials and methods
Rats were trained to self-administer either 10% ethanol or 5% sucrose (30 min/day). The orexin-1 receptor antagonist SB334867 (0, 5, 10, 15, 20 mg/kg, i.p.) was administered 30 min before the operant self-administration sessions. After these experiments, the operant self-administration behaviors were extinguished in both the ethanol and sucrose-trained rats. Upon reaching extinction criteria, SB334867 (0, 5, 10 mg/kg, i.p.) was administered 30 min before yohimbine (0 or 2 mg/kg, i.p.). In a separate experiment, the effect of SB334867 (0, 15, or 20 mg/kg, i.p.) on general locomotor activity was determined using the open-field test.
Results
The orexin-1 receptor antagonist, SB334867 (10, 15 and 20 mg/kg) decreased operant self-administration of 10% ethanol but not 5% sucrose self-administration. Furthermore, SB334867 (5 and 10 mg/kg) significantly decreased yohimbine-induced reinstatement of both ethanol and sucrose seeking. SB334867 did not significantly affect locomotor activity measured using the open-field test.
Conclusions
The results suggest that inhibition of OX-1/Hcrt-1 receptors modulates operant ethanol self-administration and also plays a significant role in yohimbine-induced reinstatement of both ethanol and sucrose seeking in rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
The orexins (or hypocretins) are neuropeptides produced exclusively in the lateral hypothalamus (de Lecea et al. 1998; Peyron et al. 1998; Sakurai et al. 1998), an area activated by consummatory rewards (Bernardis and Bellinger 1996; Levitt and Teitelbaum 1975) and strongly linked to preferences for cues associated with food and drug reward (Harris et al. 2005). Orexin/hypocretin-containing neurons have widespread projections throughout the central nervous system (CNS; Nambu et al. 1999; Peyron et al. 1998). There are currently two identified orexin/hypocretin peptides, orexin-A/hypocretin-1 and orexin-B/hypocretin-2, and two orexin/hypocretin receptors, the orexin-1/hypocretin-1 (OX-1/Hcrt-1) receptor and the orexin-2/hypocretin-2 (OX-2/Hcrt-2) receptor (de Lecea et al. 1998; Sakurai et al. 1998). The OX-1/Hcrt-1 receptor is selective for orexin-A/hypocretin-1, while the OX-2/Hcrt-2 receptor is nonselective for both orexin-A/hypocretin-1 and orexin-B/hypocretin-2 (Sakurai et al. 1998). Although both receptors are expressed throughout the CNS, the ventral tegmental area (VTA) and locus coeruleus (LC) exhibit the highest expression levels (Sakurai 2007). Furthermore, the locus coeruleus, a key modulator of attentional state, has been shown to receive the densest innervation of orexin/hypocretin neurons (Hagan et al. 1999; Horvath et al. 1999). The orexin/hypocretin system was initially implicated in the regulation of homeostatic functions such as feeding and arousal (Hagan et al. 1999; Mignot 2001; Sakurai et al. 1998; Sutcliffe and de Lecea 2002; Willie et al. 2001). However, results from more recent studies indicate that orexins/hypocretins play a significant role in reward-related behaviors (Borgland et al. 2006; Boutrel 2006; DiLeone et al. 2003; Harris and Aston-Jones 2006; Harris et al. 2005; Lawrence et al. 2006; Narita et al. 2006).
Relapse to drug seeking is a hallmark of addictive behaviors, yet there are currently few effective treatments for preventing relapse after a protracted period of abstinence. A number of methods have been developed in animals to study reinstatement of drug seeking and have adequate validity to model aspects of relapse in humans (Epstein et al. 2006, no. 16). For example, reinstatement of drug seeking in laboratory animals is induced by environmental cues and contexts associated with drug availability, reexposure to the drug itself, and stress. These conditions have been reported to induce relapse to drug taking in humans (Epstein et al. 2006; Katz and Higgins 2003; Spanagel 2003). The orexin/hypocretin system has been shown to play a key role in cue-induced reinstatement of morphine, nicotine, and ethanol seeking (DiLeone et al. 2003; Lawrence et al. 2006; Narita et al. 2006; Pasumarthi et al. 2006) and has been proposed to play a role in footshock stress-induced reinstatement of cocaine seeking (Boutrel et al. 2005); however, the role of the orexin/hypocretin system in stress- or yohimbine-induced reinstatement of ethanol seeking is not known.
It has been shown that yohimbine, an α-2 adrenoceptor antagonist, induces a stress-like state in both humans and laboratory animals (Bremner et al. 1996a, b; Vythilingam et al. 2000). In contrast to other stressors, yohimbine activates c-fos and CRF mRNA in the same brain regions as that elicited after footshock-induced stress (Funk et al. 2006). Furthermore, the brain areas activated by yohimbine and footshock were found to be regionally specific in brain regions associated with the rewarding effects of ethanol and other substances of abuse, notably, the shell of the nucleus accumbens, basolateral and central amygdalar nuclei, and the bed nucleus of the stria terminalis (Funk et al. 2006). Footshock-induced stress has been shown to be effective in inducing reinstatement of cocaine, heroin, ethanol, and nicotine seeking (Buczek et al. 1999; Erb et al. 1996; Funk et al. 2006; Le et al. 2000; Le et al. 1998; Liu and Weiss 2002; Lu et al. 2003; Shaham et al. 1997); however, its ability to reinstate seeking of natural rewards such as sucrose has been inconsistent (Buczek et al. 1999). Based on the neurochemical data and the increasing evidence showing that yohimbine reinstates reward-seeking behavior (Funk et al. 2006; Gass and Olive 2007; Ghitza et al. 2006; Le et al. 2005; Lee et al. 2004; Marinelli et al. 2007; Nair et al. 2006; Shepard et al. 2004), we used yohimbine in the current study to induce reinstatement to determine the role of the OX-1/Hcrt-1 receptors in the reinstatement of ethanol and sucrose seeking.
Materials and methods
Subjects
Male Long–Evans rats weighing 180–200 g upon arrival (Harlan Indianapolis, IN, USA), were individually housed in ventilated Plexiglas cages in a climate-controlled room on a 12-h light–dark cycle (lights on at 7 a.m.). Operant training occurred between 8 a.m. and 12 p.m., Monday through Friday. Food and water were available ad libitum, except for short periods during initial self-administration training, as outlined below. All procedures were preapproved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with NIH guidelines for the Humane Care and Use of Laboratory Animals.
Drugs
SB334867 (1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthydrin-4-yl urea HCl; Tocris, MI, USA) was made up in a vehicle solution containing saline with 25% cyclodextrin (w/v) and 5% dimethyl sulfoxide (v/v) and was administered at doses of 5, 10, 15, or 20 mg/kg (as outlined in the methods for each experiment). Yohimbine (Sigma Aldrich, MO, USA) was made up in distilled water and administered at a dose of 2 mg/kg, in accordance with previous reinstatement studies (Gass and Olive 2007; Ghitza et al. 2006; Le et al. 2005; Marinelli et al. 2007; Nair et al. 2006; Shepard et al. 2004). Vehicle injections were administered in the same volume as the injected drug as a control condition. Sucrose solutions [5 or 10% sucrose (w/v) Fisher Scientific, NJ, USA] were prepared using filtered water. The 10% ethanol (v/v) solution was prepared using 95% ethyl alcohol (Gold Shield Chemical, Hayward, CA DSP-CA-151, USA) and filtered water. In the sucrose fade experiments, 10%, 5%, 3%, and 1% sucrose, respectively, were dissolved in 10% ethanol (w/v).
Apparatus
Self-administration testing was conducted in standard operant conditioning chambers (Coulbourn Instruments, Allentown, PA, USA) enclosed in sound-attenuating cubicles with a fan for ventilation and background white noise. Each operant conditioning chamber housed two retractable levers on the right wall (4 cm above the grid floor, 12 cm apart). A liquid dipper system was placed centrally between the two levers. A house light was present on the wall opposite the levers and remained on at all times during the operant conditioning session. Stimulus lights were present 2 cm above each lever. An apparatus to emit a tone under specific conditions for operant responding was also present. Upon correct (active) lever press(es), the stimulus light above the active lever was illuminated and accompanied by a tone for 3 s to indicate availability of reward in the dipper receptacle. The dipper port was illuminated for 10 s, while the dipper cup was available. If the cup was not actively licked during the 10 s, the cup fell, and this event was recorded as a null response. For the cup to be available again, the rat had to perform another fixed ratio. The number of reinforcers obtained per operant self-administration sessions was determined as the total number of reinforcers offered minus the null responses. Stimulus, fluid delivery, and operant responses were all controlled and recorded by computer (Coulbourn Instruments, Allentown, PA, USA) using Graphic State 2.0 software. Inactive lever presses were also recorded as a measure of general activity but had no programmed consequences.
Operant self-administration procedure
Before beginning the operant self-administration training, rats were exposed to 10% ethanol or 5% sucrose solution (depending on which test group they were assigned) as the only liquid source in their home cages for 3 days. Rats were then fluid restricted for 22 h before being placed in the operant conditioning chambers for a 14-h overnight session on an FR1 schedule of reinforcement (0.1 ml after a single lever press) with either 10% or 5% sucrose as the reinforcer for the ethanol and sucrose assigned group, respectively. During this phase, only the active lever was available for the rat to press, to facilitate learning. Initial daily training consisted of 45 min FR1 operant conditioning sessions and 2-h daily water access, with water access immediately after the training sessions. Once conditions for operant responding were established (2–4 days), rats were given free access to water in the home cage and continued on a 45-min FR1 schedule for 3–4 days. Subsequently, operant conditioning training sessions were reduced to 30 min, and the work ratio increased to an FR3 schedule of reinforcement (three active lever presses required for 0.1 ml reward). A second, inactive lever was also introduced at this time. Upon pressing the inactive lever, no reinforcer, cue light, or auditory stimuli were presented, and the event was merely recorded as a measure of nonspecific behavioral activation. For rats assigned to the 10% ethanol group, a sucrose fading technique began at this point, and 10% ethanol was added to the sucrose solution (Samson 1986). Throughout the next eight to ten operant conditioning sessions, the sucrose concentration was gradually decreased (5%, 3%, and 1%) until the rats responded on an FR3 schedule for 10% ethanol alone. Stable baseline responding was achieved when the number of responses for each rat varied within 20% of the average for the group over three consecutive sessions. Both ethanol and sucrose groups had reached stable baseline responding at the FR3 level after 20 sessions.
Experiment 1—effect of SB334867 on operant self-administration of ethanol and sucrose
Rats were trained to self-administer either 10% ethanol or 5% sucrose in operant conditioning chambers as outlined above. Once the rats had achieved stable baseline responding levels, they were tested once per week with SB334867 (5, 10, 15, 20 mg/kg i.p.) or vehicle. Drug (or vehicle) was administered 30 min before the start of the operant conditioning session. All ethanol self-administering rats received all drug doses and vehicle conditions in a counterbalanced, Latin square design. In sucrose self-administering rats, initial indications showed no effect of SB334867 at 20 mg/kg, so rats were only tested with this dose or vehicle. Regular 30 min operant self-administration sessions were run on days between test sessions.
Experiment 2—effect of SB334867 on general locomotor activity
Testing was conducted using 40 × 40 cm open-field locomotor activity-monitoring chambers equipped with horizontal photobeams (Med Associates, St Albans, VT, USA). Horizontal locomotor activity was monitored at 100 ms, and stereotypic behaviors were also recorded throughout the session. Locomotor studies were run in daily 2 h sessions over four consecutive days. On day 1, rats were placed into the activity chamber for 2 h, no data were collected, and this session was used solely for habituation purposes. On days 2 and 3, animals were placed into the activity chamber, and the session was paused after 60 min, while animals were removed from the chamber and given a single i.p. injection of saline (3 ml/kg) to habituate animals to the injection procedure before the test day. Animals were returned to the activity chambers and the session continued for an additional 60 min. Data from the third day was used to assign animals to one of three treatment groups (0, 15, 20 mg/kg SB334867), six animals per group. On day 4, the animals were once again placed in the activity chambers, and the session was paused after 60 min, while the animals received a single i.p. injection of either vehicle or drug, SB334867 (15 or 20 mg/kg). The animals were returned to the activity chamber immediately after the injections, and the session continued for an additional 60 min, drug challenge. At the end of each session, animals were returned to the home cage. Data were collected across the entire 2 h (120 min) session and recorded as distance traveled in centimeters and total number of stereotypic counts. Data were separated into habituation (0–60 min) and drug challenge (60–120 min) for statistical analysis. Data analysis was performed by one-way analysis of variance (ANOVA) using the between subjects factor of treatment (SB334867 dose).
Experiment 3—effect of SB334867 on yohimbine-induced reinstatement
For the reinstatement study, ethanol- or sucrose-seeking behavior was extinguished in rats trained to self-administer 10% ethanol or 5% sucrose, respectively, under FR3 conditions. During the extinction training, lever pressing on the active lever resulted in light and tone cue presentation but no reward delivery. Neither 10% ethanol nor 5% sucrose was available throughout extinction training. Extinction training continued until the rats responded with less than ten active lever presses per session or less than 10% of their previous baseline pressing on the active lever for two consecutive sessions (Fig. 2). The rats were assigned to one of six groups (three ethanol groups and three sucrose groups), matched for their previous operant self-administration responding. We used the between subjects factor of SB334867 dose (0, 5, 10 mg/kg i.p.) and the within subjects factor of yohimbine dose (0, 2 mg/kg, i.p.) to assess the effect of SB334867 on yohimbine-induced reinstatement. Animals received two injections per test day as outlined in Table 1. On test days, the rats were first injected with either SB334867 or its vehicle and 30 min later with either yohimbine or the yohimbine vehicle. Rats were placed into the operant self-administration chambers 30 min after the second injection for the 30-min-long reinstatement test session. Reinstatement sessions were run under the same conditions as the extinction sessions; successful FR3 responses at the previously active lever resulted in light and tone cue presentation with no reward delivery. Again, inactive lever responding had no programmed consequences and was used as a measure of nonspecific behavioral activation. Rats were tested with vehicle and yohimbine consecutively over two test sessions, 7 days apart. Regular 30-min extinction sessions were run on the between-test days.
Statistics
The data from the ethanol and sucrose groups in the operant self-administration and reinstatement experiments were analyzed separately. The data from the operant self-administration studies were analyzed by repeated measures two-way ANOVA using the within-subjects factors of SB334867 dose and lever. The data from the reinstatement studies were analyzed by two-way ANOVA using the between subjects factor of SB334867 dose and within subjects factors of yohimbine dose. Active and inactive lever data were analyzed separately, as there was no effect of either SB334867 or yohimbine on inactive lever pressing and therefore no need for determination of any drug × lever interactions. For the locomotor activity study, drug challenge (60–120 min) data were analyzed by one-way ANOVA using the between subjects factor of SB334867 dose. All statistical analyses were performed using SigmaStat software, and in all cases, post hoc analysis was determined by Student Newman–Keuls test where overall significance was p < 0.05.
Results
Experiment 1: effect of SB334867 on operant self-administration of ethanol and sucrose
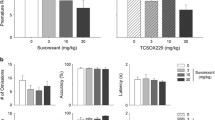
SB334867 significantly attenuated operant self-administration of 10% ethanol in a dose-dependent manner compared to vehicle-treated animals (Fig. 1a). Repeated measures two-way ANOVA of lever pressing revealed a significant effect of SB334867 dose (F[4,69] = 4.4, p < 0.01, Fig. 1a), a significant effect of lever (F[1,69] = 31.7, p < 0.001), and a significant interaction of SB334867 dose × lever (F[4,69] = 4.5, p < 0.01). Post hoc analysis further revealed that the SB334867 dose had no effect on inactive lever pressing (Table 2). One-way ANOVA analysis of number of reinforcers obtained also revealed an overall main effect of the SB334867 dose (F[4,38] = 3.0, p < 0.05). Post hoc analyses revealed that 10, 15, and 20 mg/kg doses significantly reduced the number of reinforcers obtained compared to vehicle (Fig. 1b). In contrast, SB334867 (20 mg/kg) did not affect 5% sucrose operant self-administration when compared to vehicle-treated animals (data not shown). Repeated measures two-way ANOVA of lever pressing revealed a significant effect of lever (F[1,31] = 169.5, p < 0.001), but nonsignificant effect of SB334867 dose, and no interaction of SB334867 dose × lever (data not shown). Furthermore, one-way ANOVA of the number of sucrose reinforcers also showed a nonsignificant effect of SB334867 dose (data not shown).
Effect of SB334867 on operant self-administration of 10% ethanol. a Mean ± SEM number of active lever presses. b Mean ± SEM number of ethanol reinforcers obtained. Rats were pretreated with SB334867 or its vehicle 30 min before the 30-min self-administration sessions (n = 8–9 per group). *p < 0.05; **p < 0.01; ***p < 0.001 compared to vehicle condition
Experiment 2: effect of SB334867 on general locomotor activity
There was no statistical difference in baseline general locomotor activity between treatment or vehicle groups during the 1-h habituation period. Furthermore, there was no overall main effect on the general locomotor activity during the 1-h drug challenge period that followed immediately after the SB334867 (15 or 20 mg/kg) or vehicle injection. One-way ANOVA of data during the drug challenge period revealed no effect of SB334867 on either total distance traveled or total number of stereotypic counts (data not shown).
Experiment 3: effect of SB334867 on yohimbine-induced reinstatement of ethanol and sucrose seeking
Ethanol and sucrose self-administering animals extinguished their lever pressing behavior at similar rates, requiring approximately 4 weeks to reach extinction criteria (Fig. 2). After extinction of the ethanol-seeking behavior, yohimbine (2 mg/kg, i.p.) induced reinstatement of lever responding on the active lever that had previously been associated with ethanol reinforcement. Two-way ANOVA analysis of active lever responding in ethanol-experienced rats revealed a significant interaction of yohimbine dose × SB334867 dose (F(2,57) = 5.8, p < 0.01). Post hoc analysis further revealed that yohimbine, in the absence of SB334867, significantly increased active lever responding (p < 0.001, Fig. 3). Furthermore, the reinstatement was inhibited by pretreatment with SB334867 (5 and 10 mg/kg), as both doses tested significantly attenuated yohimbine-induced reinstatement of active lever responding (Fig. 3).
Effect of SB334867 on yohimbine-induced reinstatement of ethanol seeking. Mean ± SEM number of active lever presses during extinction and reinstatement tests. Extinction is shown as the average number of lever presses across the two sessions preceding the first reinstatement test for all rats. Rats were pretreated with SB334867 or its vehicle 30 min before yohimbine or its vehicle. Yohimbine (or vehicle) was administered 30 min before the start of the reinstatement session (n = 8–11 per group). †p < 0.001 compared to yohimbine-vehicle condition in rats injected with the SB334867 vehicle (0 dose); ***p ≤ 0.001 compared to SB334867 vehicle condition in rats injected with yohimbine
In accordance with the ethanol data, two-way ANOVA analysis of active lever responding in sucrose-experienced rats revealed a significant effect of the yohimbine dose (F[1, 39] = 5.0, p < 0.05) and a significant interaction of the yohimbine dose × SB334867 dose (F[2, 39] = 6.0, p < 0.01). Post hoc analysis further revealed that yohimbine, in the absence of SB334867, significantly increased active lever responding (p < 0.001, Fig. 4). Furthermore, SB334867 significantly attenuated yohimbine-induced reinstatement of active lever responding at both doses tested (Fig. 4).
Effect of SB334867 on yohimbine-induced reinstatement of sucrose seeking. Mean ± SEM number of active lever presses during extinction and reinstatement tests. Extinction is shown as the average number of lever presses across the two sessions preceding the first reinstatement test for all rats. Rats were pretreated with SB334867 or its vehicle 30 min before yohimbine, or its vehicle. Yohimbine (or vehicle) was administered 30 min before the start of the reinstatement session (n = 6–7, per group). †p < 0.001 compared to yohimbine-vehicle condition in rats injected with the SB334867 vehicle (0 dose); **p < 0.01; ***p < 0.001 compared to SB334867 vehicle condition in rats injected with yohimbine
After extinction of the ethanol- and sucrose-seeking behavior, SB334867 alone, in the absence of yohimbine, had no effect on active lever responding compared to vehicle in either ethanol- or sucrose-experienced rats (Figs. 3 and 4, respectively). Inactive lever responses were unaffected by any treatment in both ethanol- and sucrose-experienced rats (Table 3).
Discussion
The present study shows that the OX-1/Hcrt-1 receptor antagonist, SB334867, dose dependently attenuates operant self-administration of ethanol, but not sucrose. After extinction of the ethanol- and sucrose-seeking behavior, we found that yohimbine induced a robust reinstatement of both ethanol and sucrose seeking that was significantly attenuated by SB334867 in both the ethanol- and sucrose-experienced rats.
Role of OX-1/Hcrt-1 receptors in operant self-administration of ethanol and sucrose
The OX-1/Hcrt-1 receptor antagonist SB334867 dose dependently attenuates self-administration of ethanol, but not sucrose, supporting previous studies demonstrating a role for OX-1/Hcrt-1 receptors in ethanol self-administration (Lawrence et al. 2006). The fact that SB334867 only attenuated self-administration of ethanol suggests that daily exposure to ethanol in combination with the presentation of the cues that signal ethanol availability leads to an increased activation of OX-1/Hcrt-1 receptors. This suggestion is further supported by the studies showing that orexin/hypocretin neurons are activated by ethanol-associated stimuli (Dayas et al. 2008). However, the finding that SB334867 had no effect on sucrose self-administration suggests that OX-1/Hcrt-1 receptors are not activated by sucrose or sucrose-associated cues. It remains to be determined if SB334867 reduced ethanol self-administration as a result of interference with ethanol-induced activation of OX-1/Hcrt-1 receptors and or the ethanol-associated cues.
Role of OX-1/Hcrt-1 receptors in yohimbine-induced reinstatement of ethanol and sucrose seeking
In the present study, we show that the OX-1/Hcrt-1 receptor antagonist, SB334867, attenuates yohimbine-induced reinstatement of ethanol and sucrose seeking. The protocol developed for studying reinstatement of drug seeking in animals has been shown to have validity for studying relapse to drug addiction in humans (Epstein et al. 2006; Katz and Higgins 2003; Spanagel 2003). Stress and reexposure to cues or to the context previously associated with drug availability are common reasons for relapse to drug seeking in humans and induce reinstatement of drug seeking in rodents (Liu and Weiss 2003; Shaham et al. 2000; Zironi et al. 2006). A “stress response” is generally believed to involve the CRF system and activation of the HPA-axis (for review see Koob 1999). However, emerging evidence suggests that the orexin/hypocretin system may also be involved in the response to stress (Paneda et al. 2005; Winsky-Sommerer et al. 2005). For example, the orexin/hypocretin peptides have been shown to induce dose-dependent activation of the HPA system; an effect that can be completely prevented using a CRF-1 receptor antagonist (Jaszberenyi et al. 2000), and recent data suggest that the CRF system directly innervates orexin/hypocretin expressing neurons (Winsky-Sommerer et al. 2005). Furthermore, SB334867 has been shown to attenuate footshock-induced reinstatement of cocaine seeking (Boutrel et al. 2005). Orexin-A/hypocretin-1 has been shown to induce noradrenaline release (Hirota et al. 2001) and orexin-A/hypocretin-1 induced reinstatement of cocaine seeking has been blocked using CRF-1 and noradrenergic receptor antagonists (Boutrel et al. 2005). It is also hypothesized that orexin/hypocretin neurons and CRF-expressing neurons constitute a feedback system which regulates arousal in response to stressful stimuli (Paneda et al. 2005; Winsky-Sommerer et al. 2003; 2005; Winsky-Sommerer et al. 2004).
The exact mechanism underlying yohimbine-induced reinstatement is still unknown; however, it is plausible to suggest that yohimbine may induce reinstatement through a mechanism similar to that of orexin-A/hypocretin-1. Evidence to directly support this hypothesis comes from the observation that orexin-A-/hypocretin-1-induced reinstatement and yohimbine-induced reinstatement can both be blocked by a CRF-1 receptor antagonist (Boutrel et al. 2005; Marinelli et al. 2007). In addition, yohimbine-induced increases in ethanol operant self-administration are inhibited by antalarmin, a CRF-1 receptor antagonist (Marinelli et al. 2007). Furthermore, yohimbine-induced reinstatement of palatable food seeking is reduced by inhibiting CRF-1 receptors (Ghitza et al. 2006). Both yohimbine and CRF induce release of noradrenaline in the locus coeruleus (Chen et al. 1992). Yohimbine has also been shown to up-regulate CRF expression in the central nucleus of the amygdala (Funk et al. 2006), and there is evidence to suggest that the orexin/hypocretin system can act through the amygdala to augment arousal and evoke behavioral responses associated with fear, stress, or emotion (Bisetti et al. 2006). We hypothesize that yohimbine induces a stress-like response that leads to activation of OX-1/Hcrt-1 receptors. Therefore, inhibiting OX-1/Hcrt-1 receptors attenuates the stress-like response leading to a reduction in yohimbine-induced reinstatement of reward seeking as seen in the present study.
Methodological considerations
There are two main methodological considerations regarding the present study. Firstly, it is possible that the observed decrease in operant self-administration of ethanol and the blockade of yohimbine-induced reinstatement were due to motor deficits. Secondly, the attenuated yohimbine-induced reinstatement of lever pressing could be due to nonspecific decreases in general locomotor behavior. However, there was no decrease in operant self-administration of sucrose after administration of the highest does of SB334867. In addition, SB334867 had no effect on general locomotor activity in the open field locomotor activity test. Therefore, the inhibitory effect of SB334867 on ethanol self-administration and yohimbine-induced reinstatement does not appear to be based on reduced locomotor activity. Furthermore, in accordance with previous studies (Ghitza et al. 2006; Le et al. 2005; Shepard et al. 2004), there was no increase in responding at the inactive lever after the yohimbine injection in the reinstatement experiment, suggesting that the effect of the yohimbine treatment was specific to the active lever previously associated with the reward of ethanol or sucrose, respectively.
Conclusions
The results presented in the current study demonstrate that OX-1/Hcrt-1 receptors are involved in mediating operant self-administration of ethanol. These results support previous studies demonstrating a role for OX-1/Hcrt-1 receptors in ethanol self-administration and olfactory-cue-induced reinstatement (Lawrence et al. 2006). We further show that inhibiting OX-1/Hcrt-1 receptors attenuates yohimbine-induced reinstatement of both ethanol and sucrose seeking. Our findings suggest that OX-1/Hcrt-1 receptors may represent a possible target for the treatment of alcohol use disorders.
References
Bernardis LL, Bellinger LL (1996) The lateral hypothalamic area revisited: ingestive behavior. Neurosci Biobehav Rev 20:189–287
Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, Muhlethaler M (2006) Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience 142:999–1004
Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A (2006) Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49:589–601
Boutrel B (2006) Hypocretins: between desire and needs... toward the understanding of a new hypothalamic brain pathway involved in motivation and addiction]. Med Sci (Paris) 22:573–575
Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L (2005) Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A 102:19168–19173
Bremner JD, Krystal JH, Southwick SM, Charney DS (1996a) Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse 23:28–38
Bremner JD, Krystal JH, Southwick SM, Charney DS (1996b) Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse 23:39–51
Buczek Y, Le AD, Wang A, Stewart J, Shaham Y (1999) Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 144:183–188
Chen MF, Chiu TH, Lee EH (1992) Noradrenergic mediation of the memory-enhancing effect of corticotropin-releasing factor in the locus coeruleus of rats. Psychoneuroendocrinology 17:113–124
Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F (2008) Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry 63:152–157
de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95:322–327
DiLeone RJ, Georgescu D, Nestler EJ (2003) Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73:759–768
Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1–16
Erb S, Shaham Y, Stewart J (1996) Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 128:408–412
Funk D, Li Z, Le AD (2006) Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: relationship to the reinstatement of alcohol seeking. Neuroscience 138:235–243
Gass JT, Olive MF (2007) Reinstatement of ethanol-seeking behavior following intravenous self-administration in Wistar rats. Alcohol Clin Exp Res 31:1441–1445
Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y (2006) The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology 31:2188–2196
Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N (1999) Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A 96:10911–10916
Harris GC, Aston-Jones G (2006) Arousal and reward: a dichotomy in orexin function. Trends Neurosci 29:571–577
Harris GC, Wimmer M, Aston-Jones G (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559
Hirota K, Kushikata T, Kudo M, Kudo T, Lambert DG, Matsuki A (2001) Orexin A and B evoke noradrenaline release from rat cerebrocortical slices. Br J Pharmacol 134:1461–1466
Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN (1999) Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol 415:145–159
Jaszberenyi M, Bujdoso E, Pataki I, Telegdy G (2000) Effects of orexins on the hypothalamic-pituitary-adrenal system. J Neuroendocrinol 12:1174–1178
Katz JL, Higgins ST (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 168:21–30
Koob GF (1999) Stress, corticotropin-releasing factor, and drug addiction. Ann N Y Acad Sci 897:27–45
Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006) The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148:752–759
Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y (1998) Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 135:169–174
Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y (2000) The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 150:317–324
Le AD, Harding S, Juzytsch W, Funk D, Shaham Y (2005) Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 179:366–373
Lee B, Tiefenbacher S, Platt DM, Spealman RD (2004) Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology 29:686–693
Levitt DR, Teitelbaum P (1975) Somnolence, akinesia, and sensory activation of motivated behavior in the lateral hypothalamic syndrome. Proc Natl Acad Sci U S A 72:2819–2823
Liu X, Weiss F (2002) Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci 22:7856–7861
Liu X, Weiss F (2003) Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology (Berl) 168:184–191
Lu L, Shepard JD, Hall FS, Shaham Y (2003) Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev 27:457–491
Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD (2007) The CRF(1) receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl)(195):(345–355)
Mignot E (2001) A commentary on the neurobiology of the hypocretin/orexin system. Neuropsychopharmacology 25:S5–13
Nair SG, Gray SM, Ghitza UE (2006) Role of food type in yohimbine- and pellet-priming-induced reinstatement of food seeking. Physiol Behav 88:559–566
Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K (1999) Distribution of orexin neurons in the adult rat brain. Brain Res 827:243–260
Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T (2006) Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci 26:398–405
Paneda C, Winsky-Sommerer R, Boutrel B, de Lecea L (2005) The corticotropin-releasing factor-hypocretin connection: implications in stress response and addiction. Drug News Perspect 18:250–255
Pasumarthi RK, Reznikov LR, Fadel J (2006) Activation of orexin neurons by acute nicotine. Eur J Pharmacol 535:172–176
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015
Sakurai T (2007) The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8:171–181
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:1 page following 696
Samson HH (1986) Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res 10:436–442
Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J (1997) Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci 17:2605–2614
Shaham Y, Erb S, Stewart J (2000) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev 33:13–33
Shepard JD, Bossert JM, Liu SY, Shaham Y (2004) The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 55:1082–1089
Spanagel R (2003) Alcohol addiction research: from animal models to clinics. Best Pract Res Clin Gastroenterol 17:507–518
Sutcliffe JG, de Lecea L (2002) The hypocretins: setting the arousal threshold. Nat Rev Neurosci 3:339–349
Vythilingam M, Anderson GM, Owens MJ, Halaszynski TM, Bremner JD, Carpenter LL, Heninger GR, Nemeroff CB, Charney DS (2000) Cerebrospinal fluid corticotropin-releasing hormone in healthy humans: effects of yohimbine and naloxone. J Clin Endocrinol Metab 85:4138–4145
Willie JT, Chemelli RM, Sinton CM, Yanagisawa M (2001) To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 24:429–458
Winsky-Sommerer R, Boutrel B, De Lecea L (2003) The role of the hypocretinergic system in the integration of networks that dictate the states of arousal. Drug News Perspect 16:504–512
Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L (2004) Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci 24:11439–11448
Winsky-Sommerer R, Boutrel B, de Lecea L (2005) Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol 32:285–294
Zironi I, Burattini C, Aicardi G, Janak PH (2006) Context is a trigger for relapse to alcohol. Behav Brain Res 167:150–155
Acknowledgements
This work was supported by funding from the State of California for Medical Research through UCSF to A.B. and S.E.B and Department of Defense Grants W81XWH-06-1-0240 to S.E.B and W81XWH-07-1-0429 to A.B. NARSAD Young Investigator Awards to both S.E.B and S.L.B. We also thank Dr. T.M. Gill for his help with the manuscript preparation and Haley Pierson and Brian Medina for technical support. The experiments contained herein comply with the current laws of the United States of America. All procedures were preapproved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with NIH guidelines for the Humane Care and Use of Laboratory Animals.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Principal Editor: Klaus A. Miczek, Ph.D.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Richards, J.K., Simms, J.A., Steensland, P. et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long–Evans rats. Psychopharmacology 199, 109–117 (2008). https://doi.org/10.1007/s00213-008-1136-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1136-5