Abstract
Rationale
Cocaine users have increased regional brain mu-opioid receptor (mOR) binding which correlates with cocaine craving. The relationship of mOR binding to relapse is unknown.
Objective
To evaluate regional brain mOR binding as a predictor of relapse to cocaine use is the objective of the study.
Materials and methods
Fifteen nontreatment-seeking, adult cocaine users were housed on a closed research ward for 12 weeks of monitored abstinence and then followed for up to 1 year after discharge. Regional brain mOR binding was measured after 1 and 12 weeks using positron emission tomography (PET) with [11C]carfentanil (a selective mOR agonist). Time to first cocaine use (lapse) and to first two consecutive days of cocaine use (relapse) after discharge was based on self-report and urine toxicology.
Results
A shorter interval before relapse was associated with increased mOR binding in frontal and temporal cortical regions at 1 and 12 weeks of abstinence (Ps < 0.001) and with a lesser decrease in binding between 1 and 12 weeks (Ps < 0.0008). There were significant positive correlations between mOR binding at 12 weeks and percent days of cocaine use during first month after relapse (Ps < 0.002). In multiple linear regression analysis, mOR binding contributed significantly to the prediction of time to relapse (R 2 = 0.79, P < 0.001), even after accounting for clinical variables.
Conclusions
Increased brain mOR binding in frontal and temporal cortical regions is a significant independent predictor of time to relapse to cocaine use, suggesting an important role for the brain endogenous opioid system in cocaine addiction.
Similar content being viewed by others
Introduction
There is no broadly effective treatment for cocaine addiction (Gorelick 2003; Karila et al. 2008; Knapp et al. 2007; Simpson et al. 2002). Patients vary widely in their treatment response. This situation has generated a search for markers of treatment response (Ciraulo et al. 2003). Such a marker would allow limited treatment resources to be directed towards patients who could benefit the most.
A variety of psychosocial and cocaine use variables have been found predictive of treatment response at a group level in small-scale studies, including duration of cocaine use, severity of addiction, employment status, and education level (Ciraulo et al. 2003; Hser et al. 1999; Kampman et al. 2002; McKay et al. 1999; McMahon 2001; Poling et al. 2007; Reiber et al. 2002). The search for biological prognostic markers has also had limited success (Elkashef and Vocci 2003). EEG patterns (Prichep et al. 1999), P300 event-related brain potentials (Bauer 1997), and peripheral measures of dopamine and serotonin function (Patkar et al. 2003, 2004) have been predictive of relapse by cocaine addicts in treatment in small studies, but have not been replicated in larger samples or by other research groups.
We present data in support of brain mu-opioid receptor (mOR) binding as a prognostic biomarker. Animal studies show that chronic, intermittent cocaine administration (“binge” pattern) increases mOR binding (Branch et al. 1992; Hammer 1989; Unterwald et al. 2001, 1994) and expression of mRNA for mOR (Azaryan et al. 1996a, b; Yuferov et al. 1999) in specific brain regions. We have previously reported, using positron emission tomography (PET) with the radiolabeled, selective mOR agonist [11C]carfentanil, that human cocaine users show increased mOR binding in some brain regions (e.g., frontal and lateral temporal cortex, anterior cingulate) and that this increased binding is positively correlated with self-reported cocaine craving (Zubieta et al. 1996; Gorelick et al. 2005). mOR binding increases over the first week of monitored abstinence on a research ward, then declines toward normal values over the next 12 weeks of abstinence (Gorelick et al. 2005). We report here that the degree of decline in mOR binding among subjects in the previous study is positively correlated with time to relapse to cocaine use after ward discharge, i.e., the greater the decline in mOR binding, the longer the time before relapse.
Methods and materials
Subjects
Subjects were adult, nontreatment-seeking cocaine users recruited from the community. Subject eligibility criteria included current cocaine abuse or dependence by DSM-IV criteria (American Psychiatric Association 1994); current use of cocaine averaging at least 1 g per week over the prior 3 months and at least 10 days over the prior 2 weeks; urine drug tests positive for cocaine and negative for other drugs; no clinically significant current abnormalities on the screening evaluation; no history of CNS disease, head injury with loss of consciousness greater than 3 min, or adverse event from prior cocaine use; no current psychiatric or substance use disorder except for cocaine or tobacco; not currently taking psychoactive medication; no use of opiates more than three times in the prior 3 months; IQ ≥ 80. The exclusion of subjects with other current substance use disorders or more than minimal recent opiate use was designed to minimize effects on brain mOR binding from other substance use.
Applicants received a comprehensive medical and psychological evaluation, including medical history and physical examination, clinical laboratory tests, 12-lead ECG with 3-min rhythm strip, structural magnetic resonance imaging (MRI) of the brain, Addiction Severity Index (ASI) (McLellan et al. 1985), Diagnostic Interview Schedule (Robins et al. 1982), and Symptom Check List-90R (SCL-90R; Derogatis 1983).
The study was approved by the Institutional Review Boards of the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) and the Johns Hopkins Bayview Medical Center. All subjects gave written informed consent (when not in acute drug intoxication or withdrawal) and were paid for their study participation.
Procedures
Subjects spent 3 months on the NIDA IRP closed research ward. There were usually two to three study subjects on the ward at any time, along with subjects from other studies. To prevent unauthorized use of drugs, no passes or visitors were allowed, personal belongings and mail were screened, and urine samples for drug testing were collected at random. No counseling, group therapy, or formal drug abuse treatment was provided. Subjects had access to cable television, video games, movies on videocassette, books, magazines, exercise equipment, arts and crafts supplies, and an enclosed court yard. While on the ward, subjects had PET scans done on three separate days: 1 day after ward admission (after 2 days for one subject, same day for one subject), about 1 week (6–10 days) after admission (after 18 days for one subject), and about 12 weeks (80–87 days) after admission (after 13 weeks for one subject). Subjects were not allowed to smoke for at least 30 min before each PET scan. Measures of self-reported mood (SCL-90R subscales for depression and anxiety) were obtained before each PET scan.
Follow-up measures
After discharge from the ward, subjects were followed for up to 1 year: weekly for 4 weeks, monthly for the next 5 months, then at 9 and 12 months after discharge. Subjects were paid by check for each follow-up visit according to the following schedule: $40 per visit during the first 2 months, $60 per visit during the next 3 months, and $80 per visit during the last 6 months. At each visit, subjects reported the dollar amount of their cocaine use for each day since the previous visit, using a timeline follow-back method on a blank calendar (Sobell and Sobell 1992) and provided an observed urine sample for immediate on-site assay of the cocaine metabolite benzoylecgonine (Express Test no. 93007, Biosite, San Diego, CA, USA). To maximize the validity of self-report, urine samples were obtained first, information was elicited by trained research staff in a private room when subjects were not clinically intoxicated, subjects were informed of the steps taken to ensure confidentiality (including a federal Certificate of Confidentiality for the study), and there were no adverse consequences for self-reported drug use or a positive urine drug test (Brown et al. 1992; Hamid et al. 1999; Harrison 1995; Nelson et al. 1998).
Several lapse/relapse variables were calculated from the self-report data (Donovan 1996): time to lapse = number of days from ward discharge until first cocaine use; time to relapse = number of days from ward discharge until two consecutive days of cocaine use; percent days of cocaine use during the 30 days following relapse (= number of days with cocaine use divided by total number of days with data). While there is no generally accepted operational definition of relapse in the drug abuse field (Maisto and Connors 2006), it is usually defined in terms of more extended use after a lapse that reflects loss of control over use (Donovan 1996; Milby et al. 2004). For this study, we defined a relapse as two consecutive days of cocaine use, on the grounds that this would be a reliable retrospective self-report measure of use reflecting some loss of control.
The validity of subjects’ self-report about cocaine use was evaluated by comparing urine drug test results with the self-reports for the 3 days preceding the sampling day [the typical duration of persistence of benzoylecgonine in urine (Cone et al. 2003; Preston et al. 2002)]. There was 80% overall agreement between subjects’ self-report of cocaine use and results of the urine drug tests, comparable to levels of agreement reported in large published studies (Brady et al. 2002; Brown et al. 1992; Harrison 1995; Hersh et al. 1999; Nelson et al. 1998).
PET image acquisition
PET scans were acquired in 2D mode on a GE 4096 Plus PET scanner (GE Medical Systems, Milwaukee, WI) using [11C]carfentanil, a specific mu-opioid agonist developed for PET imaging (Frost et al. 1985). Images were simultaneously collected from 15 contiguous planes with an axial field-of-view of 10.5 cm. A transmission scan of 10-min duration was obtained using rotating germanium-68 rods before injection of the radiotracer. After intravenous bolus administration of 19.5 (1.2) mCi (range = 16.3–21.1 mCi) of [11C]-carfentanil (specific activity = 3,213 ± 2,351 mCi/μmol, range = 1,475–11,090 mCi/μmol), 25 sets of images with variable time period (6 × 30 s, 5 × 60 s, 5 × 120 s, 9 × 480 s) were acquired during a 90-min period for each study. After correction for attenuation using the transmission scan, images were reconstructed in a 128 × 128 × 15 matrix with pixel size of 2 × 2 × 6.5 mm with filtered back projection methods using a ramp filter and decay-corrected.
Parameterized images for [11C]-carfentanil were generated using an automated in-house program based on Logan graphical analysis (Logan et al. 1996) with a reference-tissue model using the occipital cortex. The fitted linear slope (10–90 min) generates the ratio of the total distribution (DVR) in receptor-rich region and reference tissue. As previously described, the occipital cortex value of k 2 was assumed to be 0.104 min−1 (Endres et al. 2003). \({\text{DVR}} = {{{\text{DV}}_{{\text{rc}}} } \mathord{\left/ {\vphantom {{{\text{DV}}_{{\text{rc}}} } {{\text{DV}}_{{\text{occ}}} }}} \right. \kern-\nulldelimiterspace} {{\text{DV}}_{{\text{occ}}} }} = \left( {1 + {{k_3 } \mathord{\left/ {\vphantom {{k_3 } {k_4 }}} \right. \kern-\nulldelimiterspace} {k_4 }}} \right)\), in which k 3/k 4 represents the receptor-binding potential (BP). Parameterized images of BP were computed from BP = DVR − 1.
Data analyses
All scan data and parametric images were processed using SPM2 (The Welcome Department of Cognitive Neurology, Institute of Neurology, University College London) implanted in Matlab 6.5 (Mathworks, Natick, MA, USA) and Analyze™ software packages (Ver 6.0, Mayo Foundation, Rochester, MN).
The 0- to 90-min average image of each study was created to provide more anatomical information for spatial normalization. The average images were spatially normalized to a [11C]-carfentanil template into a standard space (MNI, Montreal Neurological Institute). The transformation matrix provided was then applied to the corresponding parametric images. Data were then smoothed using a 10-mm FWHM Gaussian smoothing kernel to reduce residual intersubject anatomical differences, increase the signal-to-noise ratio at the expense of resolution, and increase the validity of the statistical inferences by rendering the data more normally distributed.
The smoothed BP images were proportionally scaled to the global brain-binding value to yield normalized-binding potential of mOR. Proportional scaling allows identification of changes in regional mOR binding that exceed those in global mean mOR binding. The mean global binding was computed on a subset of data > whole mean/8. This had the effect of removing regions of very low or negative mOR binding. The analysis threshold was set to >70% of the global mean computed by SPM2 for each subject in order to retain in the analysis only the specific mOR binding.
The relationship of regional mOR binding to lapse/relapse variables was assessed at each voxel using the general linear model in SPM2. Because of the skewed distribution of these clinical variables, they were analyzed as log transforms. The relationship between changes in mOR binding during enforced abstinence (day 1 or weeks 1 to 12) and lapse/relapse variables or between mOR binding at a particular time point (week 1 or 12) and lapse/relapse variables was evaluated with simple correlation analysis using the “Multi-subjects: conditions and covariate” option for voxel-based analysis. A correlation was considered significant if a cluster consisting of at least 50 contiguous voxels exceeded an uncorrected height threshold of P < 0.005 (T = 3.01). Clusters showing a significant correlation in the SPM analysis were subjected to additional analysis in SPSS (v. 11.5, SPSS, Chicago, IL, USA). The mean mOR binding in the cluster (calculated using MarsBaR software [Matthew Brett, http://marsbar.sourceforge.net]) was used in a linear regression analysis to calculate the R 2 value and p value of the correlation with lapse/relapse and to generate graphs illustrating the relationship (Figs. 1, 2, 3). As a separate secondary analysis, each cluster found to have a significant correlation with lapse/relapse in the primary SPM analysis was also evaluated for the significance of its size using a voxel-based analysis within SPM.
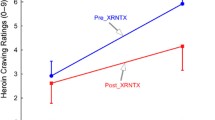
Correlation of decreases in regional brain mu-opioid receptor (mOR) binding between 1 and 12 weeks of monitored abstinence with time to relapse to cocaine use in 15 cocaine-using subjects (see Table 1). a Brain regions (in red) showing a significant positive correlation between ΔmOR binding (weeks 1–12) and time to relapse. Color bar presents the T scores. The axial plane is cut from 40 mm below the bicommissural plane at 4-mm intervals. b and c show relationship between ΔmOR binding (Y axis) and log (time to relapse) (X axis) in the right inferior frontal cortex [Brodman area 11, yellow arrow (b)] and in the right ventrolateral frontal cortex [Brodman area 47, red arrow (c)]. Each data point represents an individual subject
Correlation of regional brain mu-opioid receptor (mOR) binding after 1 or 12 weeks of monitored abstinence with time to relapse to cocaine use in 15 cocaine-using subjects (see Table 2). Brain regions (in red) showing a significant negative correlation after week 1 (a) or 12 (b) of abstinence. Color bar presents the T scores. The axial plane is cut from 24 mm below the bicommissural plane at 4 mm intervals. c and d show the relationship between mOR binding (Y axis) in the left orbitofrontal cortex (yellow arrow in a and b) after 1 week (c) or 12 weeks (d) of abstinence and log (time to relapse; X axis). Each data point represents an individual subject
Correlation of regional brain mu-opioid receptor (mOR) binding after 12 weeks of monitored abstinence with percent days of cocaine use during the month following relapse in 15 cocaine-using subjects (see Table 3). a Brain regions (in red) showing a significant positive correlation. Color bar presents the T scores. The axial plane is cut from 24 mm below the bicommissural plane at 4 mm intervals. b and c show the relationship between mOR binding (Y axis) in the left inferior frontal cortex (yellow arrow in a), b and right anterior cingulate cortex (red arrow in a), c after 12 weeks of abstinence and percent days of cocaine use (X axis) during the month after relapse. Each data point represents an individual subject
The relative value of mOR binding and subjects’ baseline clinical characteristics in predicting time to lapse and relapse was evaluated with the linear regression function of SPSS v. 11.5 (SPSS). First, univariate regression analyses were performed between subjects’ clinical characteristics (obtained from the ASI interview done at screening) and time to lapse and relapse. Clinical variables that were significant in the regression analyses were then included with regional brain mOR binding in a second round of stepwise multiple regression analyses to evaluate their relative predictive contribution (entry criterion was significance of F value < 0.001). These analyses used mean mOR binding in the most significant cluster in the voxel-based SPM analysis (Table 1), calculated with MarsBaR software (Matthew Brett, http://marsbar.sourceforge.net).
Results
Sample description
Twenty subjects enrolled in the study: one withdrew before admission because of inability to tolerate the MRI scan, one could not be studied because of technical problems in radiolabeling carfentanil, and one was discharged from the ward after 1 day because of behavioral issues. The remaining 17 subjects had at least one PET scan; their findings have been presented previously (Gorelick et al. 2005). Of these 17 subjects, one withdrew from the study prior to ward discharge and so was lost to follow-up, and one had a technically inadequate second PET scan. The remaining 15 subjects (six with cocaine dependence, nine with abuse) included in the present relapse analysis were ten African-American men, three African-American women, and two white men with mean (SD) age 33.8 (4.1) years. All subjects smoked cocaine; two also occasionally used intranasally. They had used cocaine regularly for 7.6 (4.5) years. Twelve subjects tended to use cocaine daily (subject to availability); three used cocaine intermittently (i.e., several days a week, with abstinence the other days). They smoked cocaine on 10.9 (2.7) of the 14 days prior to ward admission (three subjects reported less than 10 days of use). They reported spending $499 ($432) on cocaine during those 2 weeks. Their last cocaine use was 14.9 (10.1) h prior to admission. Use of other illegal drugs was negligible. No subject reported opiate use during the month prior to admission; all urine drug tests were negative for opiates.
As expected from the study eligibility criteria, no subject was clinically depressed at the time of admission. SCL-90R depression and anxiety subscale scores remained low throughout the study, e.g., mean (SD) of 0.8 (0.15) and 0.04 (0.10), respectively, before the first PET scan and 0.03 (0.08) and 0.006 (0.02) before the second PET scan. There were no significant correlations between subscale scores and mOR binding (Gorelick et al. 2005).
Cocaine use during follow-up
Subjects were followed after ward discharge for a mean (SD) of 28.2 (22.2) weeks (median = 28 weeks, range 1 to 52 weeks). The first cocaine use (lapse) occurred 6.1 (9.0) days (range = 0–32 days, median = 1 day) after discharge; the first relapse 67.8 (126.2) days (range = 0–363 days, median = 6 days) after discharge. The mean interval between lapse and relapse was 61.7 (125.1) days (range = 0–357 days, median = 0 days). Subjects reported using cocaine on 50.9% (38.0%) of the first 30 days after lapse, and on 55.9% (34.2%) of the first 30 days after relapse.
Brain mu-opioid receptor-binding and relapse
The decrease in mOR binding in the right inferior frontal cortex (including adjacent ventral striatum) between 1 and 12 weeks of abstinence showed a significant positive correlation with time to relapse to cocaine use after discharge (Table 1; Fig. 1), i.e., the greater the decrease in binding during abstinence, the longer the time before relapse. The right and left temporal cortex and left thalamus also had decreases in mOR binding between 1 and 12 weeks of abstinence that correlated significantly with time to relapse (Table 1; Fig. 1). There was a similar pattern of correlations between mOR binding and time to lapse (data not shown).
There were significant negative correlations between mOR binding in bilateral frontal cortex after 1 or 12 weeks of abstinence and time to relapse to cocaine use (Table 2; Fig. 2), i.e., the higher the binding, the shorter the time before relapse.
There was also a significant correlation between regional brain mOR binding after 12 weeks of monitored cocaine abstinence and severity of relapse. There was a significant positive correlation between mOR binding in the right anterior cingulate cortex and bilateral inferior frontal cortex and percent days of cocaine use during the 30 days following relapse (Table 3; Fig. 3), i.e., the higher the mOR binding, the greater the percentage of days on which cocaine was reported used.
To complement these correlational analyses, we compared regional mOR binding between the eight subjects who relapsed within 10 days of ward discharge (early relapsers) and the seven subjects who relapsed later (late relapsers). The early relapsers had significantly smaller decreases in mOR binding between weeks 1 and 12 in the right ventral striatum (max-z = 3.99 at coordinates 20, 16, −12, cluster size k = 110), right orbitofrontal cortex (max-z = 3.92 at 36, 42, −20, k = 111), right frontal cortex (max-z = 3.61 at 56, 14, 4, k = 60), and left anterior temporal cortex (max-z = 3.38 at −48, 18, −28, k = 55). The early relapsers also had greater mOR binding at week 1 in the left temporal cortex (max-z = 3.92 at −50, −24, −34, k = 93), left orbitofrontal cortex (max-z = 3.92 at −18, 56, −16, k = 62), right ventral striatum (max-z = 3.32 at 18, −4, −10, k = 69), and right temporal cortex (max-z = 3.15 at −48, 18, −28, k = 56). These findings of greater initial (week 1) mOR binding and less decrease in mOR binding between weeks 1 and 12 in the early relapsers are consistent with the correlational analyses. The latter also showed greater initial mOR binding and lesser weeks 1 to 12 decrease in mOR binding associated with shorter time to relapse.
Three subject baseline characteristics from the ASI correlated significantly with time to lapse in univariate analyses; only one ASI variable correlated significantly with time to relapse (Table 4). Other clinical variables previously reported to be significantly associated with relapse in treatment samples, e.g., days or years of cocaine use or employment status (McKay et al. 1999), did not show significant correlations with lapse or relapse in this nontreatment-seeking sample. Stepwise multiple regression analysis including the one clinical variable (years of lifetime alcohol use) with a significant univariate correlation found that a mOR-binding variable (decrease in binding in the right inferior frontal cortex between 1 and 12 weeks of abstinence) was a more significant predictor of time to relapse than was years of lifetime alcohol use (Table 4). These two variables combined accounted for 86% of the variance in time to relapse. This mOR-binding variable was second only to legal problems composite score in predicting time to lapse in a stepwise analysis including the three clinical variables (drug, legal, and family/social problems ASI composite scores) with significant univariate correlations (Table 4). These two variables combined accounted for 91% of the variance in time to lapse.
The four subject baseline characteristics from the ASI that showed significant univariate correlations with time to lapse or relapse did not show a consistent pattern of univariate correlations with regional brain mOR binding at weeks 1 and 12 (data not shown). For example, at week 1, lifetime alcohol use (years) had a significant positive correlation with mOR binding in the right inferior frontal cortex and a significant negative correlation with mOR binding in the right inferior and superior frontal cortex and left superior temporal cortex. At week 12, there were no significant positive correlations and significant negative correlations only in the left precentral frontal cortex and right inferior frontal cortex.
Discussion
We found that increased mOR binding in frontal and temporal cortex, and changes in binding over 12 weeks of monitored abstinence, are associated with time to relapse (and the related variable time to lapse) after release from monitored abstinence on a closed research ward. Regional mOR binding contributed significant predictive power beyond that provided by standard clinical variables such as drug and alcohol use and employment status.
These findings suggest that brain mOR binding could serve as a predictive marker for patient response to cocaine addiction treatment. Confirmation of this finding offers the potential to use mOR binding as a tool to identify patients at greater risk of relapse and to better allocate limited drug addiction treatment resources to patients most in need. The observation of a significant correlation between time to relapse and mOR binding 11 weeks before discharge suggests that mOR binding may have predictive value prior to or early in treatment. All significant R 2 values (square of correlation coefficient) were within the range of 0.57–0.79 (Tables 1 and 2). Our sample size was too small to provide sufficient statistical power to evaluate whether any of these were significantly higher (i.e., more predictive) than the others.
Subjects’ mood is unlikely to have confounded the observed relationship between brain mOR binding and relapse. Subjects had no current DSM-IV mood disorder and low self-rated depression or anxiety (SCL-90R subscales) throughout the study. Furthermore, there were no significant correlations between depression or anxiety scores and mOR binding (Gorelick et al. 2005).
We are aware of only two other studies, both involving subjects with alcoholism, in which a human brain imaging parameter was significantly correlated with relapse to substance use (Grusser et al. 2004; Guardia et al. 2000). In one study, detoxified alcoholic patients who relapsed during 3 months of outpatient treatment had significantly greater dopamine D2 receptor availability in the striatum [assessed by single photon emission computed tomography (SPECT) with radiolabeled iodobenzamide] during their initial inpatient detoxification than did patients who did not relapse (based on self-reported drinking status; Guardia et al. 2000). In the other study, the intensity of activation in the anterior cingulate, medial prefrontal cortex, and striatum (measured by fMRI) in response to alcohol-related cues was significantly correlated with amount of alcohol intake over the subsequent 3 months in ten alcoholic patients scanned while abstinent (Grusser et al. 2004).
The mechanism mediating the relationship between brain mOR binding and relapse cannot be directly determined from this study. Based on animal studies and our previous human studies (Zubieta et al. 1996; Gorelick et al. 2005), we suggest that increased mOR activation (due to increased binding potential) mediates increased cocaine craving, which increases the risk of relapse in many (Palij et al. 1996; Rohsenow et al. 2007; Weiss et al. 2003), but not all (Kosten et al. 2006), studies of cocaine addiction treatment. The mechanism by which mORs are upregulated is incompletely understood, but may be related to tonically reduced release of endogenous opioid receptor ligands associated with long-term cocaine exposure (Daunais et al. 1997; Laforge et al. 2003; Przewlocka and Lason 1995). In this study, two of the brain regions showing significant correlations between increased mOR binding and time to or severity of relapse were the inferior frontal cortex and anterior cingulate cortex. Activation of these brain regions has been associated with cocaine craving, salience of reinforcers, and risk of relapse in animal models of relapse to drug self-administration (Daglish and Nutt 2003; Goldstein and Volkow 2002; Kalivas and McFarland 2003). Thus, increased activation of mORs in these brain regions may have mediated the increased tendency to relapse to cocaine use among our subjects (Gianoulakis 2004). The inferior frontal cortex is an important region in brain circuits involved with memory for reinforcement and risk-reward decision-making (Robbins and Everitt 2002; Volkow et al. 2002), i.e., the ability to make decisions that appropriately balance the probability and magnitude of positive and negative consequences. The anterior cingulate cortex also has a role in risk-reward decision-making (Bush et al. 2002; Williams et al. 2004).
This study has some limitations. Subjects were not representative of all cocaine-dependent individuals because we excluded those with other current substance use disorders (except tobacco dependence) and with more than minimal recent opiate use. This was done to minimize possible effects on brain mOR binding from other substance use and give greater ability to detect a relationship between mOR binding and relapse. However, there is no reason to believe that the underlying relationship observed in our somewhat selected sample would not also hold for subjects with current substance use other than cocaine.
Subjects were not treatment-seeking and did not receive drug abuse treatment during their 3-month residential stay or 1-year follow-up. Previous studies showing an association between sociodemographic and drug use variables and relapse to cocaine use have been conducted in treatment settings with treatment-seeking patients (Ciraulo et al. 2003; Kampman et al. 2002; McKay et al. 1999; McMahon 2001; Poling et al. 2007; Reiber et al. 2002). In our sample of nontreatment-seeking subjects, a few of these clinical variables showed a significant correlation with time to lapse or relapse. This lack of correlation may be due, in part, to our relatively small sample size (15 subjects), but also could reflect a difference in valid predictors of relapse between treatment and nontreatment samples. Thus, the generalizability of our findings to treatment-seeking cocaine users in a treatment setting remains to be confirmed.
Cocaine use during follow-up was measured by subject self-report, with periodic confirmation by urine drug testing. The study procedures should have maximized valid self-report (Brown et al. 1992; Hamid et al. 1999; Harrison 1995; Nelson et al. 1998). There was 80% overall agreement between self-report and the urine drug tests, comparable to the agreement reported in large published studies (Brown et al. 1992; Harrison 1995; Hersh et al. 1999; Nelson et al. 1998; Simpson et al. 2002). In any case, distortions of self-report would have decreased the ability to detect a significant correlation with mOR binding (unless one assumes a significant association between mOR binding and tendency to underreport drug use).
The PET scanning procedure used does not distinguish between increased numbers of mOR and increased receptor affinity. This limits the ability to deduce the mechanism of the observed effect. Animal studies suggest that cocaine exposure in a binge pattern increases mOR number rather than affinity (Unterwald et al. 1992).
In summary, our findings show that increased regional brain mOR binding is associated with shorter time to relapse and more severe relapse to cocaine use after monitored abstinence. These findings improve our understanding of the neuropharmacological mechanisms involved in cocaine addiction and could lead to improved treatment and prediction of treatment outcome.
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington, DC
Azaryan AV, Clock BJ, Cox BM (1996a) Mu opioid receptor mRNA in nucleus accumbens is elevated following dopamine receptor activation. Neurochem Res 21:1411–1415
Azaryan AV, Coughlin LJ, Buzas B, Clock BJ, Cox BM (1996b) Effect of chronic cocaine treatment on mu- and delta-opioid receptor mRNA levels in dopaminergically innervated brain regions. J Neurochem 66:443–448
Bauer LO (1997) Frontal P300 decrements, childhood conduct disorder, family history, and the prediction of relapse among abstinent cocaine abusers. Drug Alcohol Depend 44:1–10
Brady KT, Sonne SC, Malcolm RJ, Randall CL, Dansky BS, Simpson K, Roberts JS, Brondino M (2002) Carbamazepine in the treatment of cocaine dependence: subtyping by affective disorder. Exp Clin Psychopharmacol 10:276–285
Branch AD, Unterwald EM, Lee SE, Kreek MJ (1992) Quantitation of preproenkephalin mRNA levels in brain regions from male Fischer rats following chronic cocaine treatment using a recently developed solution hybridization assay. Brain Res Mol Brain Res 14:231–238
Brown J, Kranzler HR, Del Boca FK (1992) Self-reports by alcohol and drug abuse inpatients: factors affecting reliability and validity. Br J Addict 87:1013–1024
Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR (2002) Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A 99:523–528
Ciraulo DA, Piechniczek-Buczek J, Iscan EN (2003) Outcome predictors in substance use disorders. Psychiatr Clin North Am 26:381–409
Cone EJ, Sampson-Cone AH, Darwin WD, Huestis MA, Oyler JM (2003) Urine testing for cocaine abuse: metabolic and excretion patterns following different routes of administration and methods for detection of false-negative results. J Anal Toxicol 27:386–401
Daglish MR, Nutt DJ (2003) Brain imaging studies in human addicts. Eur Neuropsychopharmacol 13:453–458
Daunais JB, Nader MA, Porrino LJ (1997) Long-term cocaine self-administration decreases striatal preproenkephalin mRNA in rhesus monkeys. Pharmacol Biochem Behav 57:471–475
Derogatis LR (1983) SCL-90R administration, scoring and procedures. Manual II. Clinical Psychometric Research, Towson, MD
Donovan DM (1996) Assessment issues and domains in the prediction of relapse. Addiction 91(Suppl):S29–S36
Elkashef A, Vocci F (2003) Biological markers of cocaine addiction: implications for medications development. Addict Biol 8:123–139
Endres CJ, Bencherif B, Hilton J, Madar I, Frost JJ (2003) Quantification of brain mu-opioid receptors with [11C]carfentanil: reference-tissue methods. Nucl Med Biol 30:177–186
Frost JJ, Wagner HN Jr, Dannals RF, Ravert HT, Links JM, Wilson AA, Burns HD, Wong DF, McPherson RW, Rosenbaum AE (1985) Imaging opiate receptors in the human brain by positron tomography. J Comput Assist Tomogr 9:231–236
Gianoulakis C (2004) Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem 4:39–50
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psych 159:1642–1652
Gorelick DA (2003) Pharmacologic interventions for cocaine, crack, and other stimulant addiction. In: Graham AW, Schultz TK, Mayo-Smith MF, Ries RK, Wilford BB (eds) Principles of addiction medicine. American Society of Addiction Medicine, Chevy Chase, MD, pp 785–800
Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, Endres CJ, Dannals RF, Frost JJ (2005) Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry 57:1573–1582
Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A (2004) Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 175:296–302
Guardia J, Catafau AM, Batlle F, Martin JC, Segura L, Gonzalvo B, Prat G, Carrio I, Casas M (2000) Striatal dopaminergic D(2) receptor density measured by [(123)I]iodobenzamide SPECT in the prediction of treatment outcome of alcohol-dependent patients. Am J Psychiatry 157:127–129
Hamid R, Deren S, Beardsley M, Tortu S (1999) Agreement between urinalysis and self-reported drug use. Subst Use Misuse 34:1585–1592
Hammer RP (1989) Cocaine alters opiate receptor binding in critical brain reward regions. Synapse 3:55–60
Harrison LD (1995) The validity of self-reported data on drug use. J Drug Issues 25(1):91–111
Hersh D, Mulgrew CL, Van Kirk J, Kranzler HR (1999) The validity of self-reported cocaine use in two groups of cocaine abusers. J Consult Clin Psychol 67:37–42
Hser YI, Maglione M, Boyle K (1999) Validity of self-report of drug use among STD patients, ER patients, and arrestees. Am J Drug Alcohol Abuse 25:81–91
Kalivas PW, McFarland K (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168:44–56
Kampman KM, Volpicelli JR, Mulvaney F, Rukstalis M, Alterman AI, Pettinati H, Weinrieb RM, O’Brien CP (2002) Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav 27:251–260
Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lepine JP (2008) New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol 11:1–14
Knapp WP, Soares BG, Farrel M, Lima MS (2007) Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database Syst Rev, Issue 3, Art. No. CD003023
Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE (2006) Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology 31:644–650
Laforge KS, Yuferov V, Zhou Y, Ho A, Nyberg F, Jeanne KM (2003) “Binge” cocaine differentially alters preproenkephalin mRNA levels in guinea pig brain. Brain Res Bull 59:353–357
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840
Maisto SA, Connors GJ (2006) Relapse in the addictive behaviors: integration and future directions. Clin Psychol Rev 26:229–231
McKay JR, Alterman AI, Mulvaney FD, Koppenhaver JM (1999) Predicting proximal factors in cocaine relapse and near miss episodes: clinical and theoretical implications. Drug Alcohol Depend 56:67–78
McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP (1985) New data from the Addiction Severity Index. Reliability and validity in three centers. J Nerv.Ment.Dis 173:412–423
McMahon RC (2001) Personality, stress, and social support in cocaine relapse prediction. J Subst Abuse Treat 21:77–87
Milby JB, Schumacher JE, Vuchinich RE, Wallace D, Plant MA, Freedman MJ, McNamara C, Ward CL (2004) Transitions during effective treatment for cocaine-abusing homeless persons: establishing abstinence, lapse, and relapse, and reestablishing abstinence. Psychol Addict Behav 18:250–256
Nelson DB, Kotranski L, Semaan S, Collier K, Lauby J, Feighan K, Halbert J (1998) The validity of self-reported opiate and cocaine use by out-of-treatment drug users. J Drug Issues 28(2):483–494
Palij M, Rosenblum A, Magura S, Handelsman L, Stimmel B (1996) Daily cocaine use patterns: effects of contextual and psychological variables. J Addict Dis 15:13–37
Patkar AA, Gottheil E, Berrettini WH, Thornton CC, Hill KP, Weinstein SP (2003) Relationship between platelet serotonin uptake sites and treatment outcome among African-American cocaine dependent individuals. J Addict Dis 22:79–92
Patkar AA, Mannelli P, Certa KM, Peindl K, Murray H, Vergare MJ, Berrettini WH (2004) Relationship of serum prolactin with severity of drug use and treatment outcome in cocaine dependence. Psychopharmacology (Berl) 176:74–81
Poling J, Kosten TR, Sofuoglu M (2007) Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse 33:191–206
Preston KL, Epstein DH, Cone EJ, Wtsadik AT, Huestis MA, Moolchan ET (2002) Urinary elimination of cocaine metabolites in chronic cocaine users during cessation. J Anal Toxicol 26:393–400
Prichep LS, Alper KR, Kowalik SC, Vaysblat LS, Merkin HA, Tom M, John ER, Rosenthal MS (1999) Prediction of treatment outcome in cocaine dependent males using quantitative EEG. Drug Alcohol Depend 54:35–43
Przewlocka B, Lason W (1995) Adaptive changes in the proenkephalin and D2 dopamine receptor mRNA expression after chronic cocaine in the nucleus accumbens and striatum of the rat. Eur Neuropsychopharmacol 5:465–469
Reiber C, Ramirez A, Parent D, Rawson RA (2002) Predicting treatment success at multiple timepoints in diverse patient populations of cocaine-dependent individuals. Drug Alcohol Depend 68:35–48
Robbins TW, Everitt BJ (2002) Limbic–striatal memory systems and drug addiction. Neurobiol Learn Mem 78:625–636
Robins LN, Helzer JE, Ratcliff KS, Seyfried W (1982) Validity of the diagnostic interview schedule, version II: DSM-III diagnoses. Psychol Med 12:855–870
Rohsenow DJ, Martin RA, Eaton CA, Monti PM (2007) Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. J Stud Alcohol Drugs 68:641–648
Simpson DD, Joe GW, Broome KM (2002) A national 5-year follow-up of treatment outcomes for cocaine dependence. Arch Gen Psychiatry 59:538–544
Sobell LC, Sobell MB (1992) Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J (eds) Measuring alcohol consumption: psychosocial and biological methods. Humana, Totowa, NJ, pp 41–72
Unterwald EM, Horne-King J, Kreek MJ (1992) Chronic cocaine alters brain mu opioid receptors. Brain Res 584:314–318
Unterwald EM, Rubenfeld JM, Kreek MJ (1994) Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport 5:1613–1616
Unterwald EM, Kreek MJ, Cuntapay M (2001) The frequency of cocaine administration impacts cocaine-induced receptor alterations. Brain Res 900:103–109
Volkow ND, Fowler JS, Wang GJ, Goldstein RZ (2002) Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem 78:610–624
Weiss RD, Griffin ML, Mazurick C, Berkman B, Gastfriend DR, Frank A, Barber JP, Blaine J, Salloum I, Moras K (2003) The relationship between cocaine craving, psychosocial treatment, and subsequent cocaine use. Am J Psychiatry 160:1320–1325
Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN (2004) Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci 7:1370–1375
Yuferov V, Zhou Y, Spangler R, Maggos CE, Ho A, Kreek MJ (1999) Acute “binge” cocaine increases mu-opioid receptor mRNA levels in areas of the rat mesolimbic mesocortical dopamine system. Brain Res Bull 48:109–112
Zubieta J-K, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ (1996) Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med 2:1225–1229
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse and NIH grant RO1 DA-09479 to JJF.
Financial Disclosures
The authors have no competing financial interests to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorelick, D.A., Kim, Y.K., Bencherif, B. et al. Brain mu-opioid receptor binding: relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology 200, 475–486 (2008). https://doi.org/10.1007/s00213-008-1225-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1225-5