Abstract
Rationale and objectives
The sensitivity to ethanol central effects is partially determined by the subunit composition of brain nicotinic acetylcholine receptors (nAChRs). Thus, the effects of intraventral tegmental area (VTA) administration of the nicotinic subunit-specific antagonist, α-conotoxin MII (αCtxMII, α3β2*, β3*, α6*), were compared to those of systemic mecamylamine (MEC, an allosteric negative modulator of the nAChR), dihydro-β-erythroidine (DHβE, α4β2*), and methyllycaconitine (MLA, α7*) to elucidate involvement of different subunits of nAChRs in operant ethanol self-administration and relapse-like activation of ethanol consumption after ethanol deprivation in rats.
Methods
The effects of drugs were studied in rats trained for operant oral self-administration of ethanol (FR = 1). For ethanol deprivation, trained animals were subjected to a period of alcohol deprivation for 10 days. αCtxMII was given directly into the VTA through implanted permanent intracranial cannulae, whereas MEC, DHβE, and MLA were administered systemically.
Results
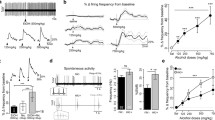
αCtxMII reduced operant ethanol self-administration and blocked the deprivation-induced relapse-like ethanol consumption. MEC reduced operant ethanol self-administration and inhibited the deprivation-induced increase in alcohol consumption. DHβE did not alter ethanol self-administration in the lower-dose range but inhibited ethanol intake at a higher dose (4 mg/kg), although this effect might have been nonspecific. MLA failed to block self-administration of ethanol and relapse-like drinking after deprivation.
Conclusions
Our results indicate that nAChRs are involved in the modulation of operant alcohol self-administration and relapse-like alcohol drinking behavior in rats. Our observations support the working hypothesis that systemically active selective ligands for nAChR α3β2*, β3, and/or α6* receptor subunits might be of therapeutic value for the treatment of alcoholism.


Similar content being viewed by others
References
Azam L, Winzer-Serhan UH, Chen Y, Leslie FM (2002) Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol 444(3):260–274
Barrett SP, Tichauer M, Leyton M, Pihl RO (2006) Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend 81(2):197–204
Benwell ME, Balfour DJ (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105:849–856
Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM (2005) Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcohol Clin Exp Res 29(3):295–302
Bowman WC, Rand MJ (1980) Textbook of pharmacology, 2nd edn. Blackwell Scientific, Oxford
Buisson B, Gopalakrishnan M, Arneric SP, Sullivan JP, Bertrand D (1996) Human _4_2 neuronal nicotinic acetylcholine receptor in HEK 293 cells: a patch clamp study. J Neurosci 16:7880–7891
Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM (1996) A new a-conotoxin which targets a3b2 nicotinic acetylcholine receptors. J Biol Chem 271:7522–7528
Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP (2002) Distribution and pharmacology of a6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci 22:1208–1217
Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23(21):7820–7829
Champtiaux N, Kalivas PW, Bardo MT (2006) Contribution of dihydro-beta-erythroidine sensitive nicotinic acetylcholine receptors in the ventral tegmental area to cocaine-induced behavioral sensitization in rats. Behav Brain Res 168(1):120–126
Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC (1997a) Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther 280(1):346–356
Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC (1997b) Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors ha2b2, ha2b4, ha3b2, ha3b4, ha4b2, ha4b4 and ha7 expressed in Xenopus oocytes. J Pharmacol Exp Ther 280:346–356
Clarke PB, Pert CB, Pert A (1984) Autoradiographic distribution of nicotine receptors in rat brain. Brain Res 323:390–395
Clarke PB, Fu DS, Jakubovic A, Fibiger HC (1988) Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther 246:701–708
Collins AC, Grady SR, Marks MJ (1995) Ethanol selectively modulates brain nicotinic receptor function. Alcohol Clin Exp Res 19:Abstract 502
Corringer PJ, Le Novere N, Changeux JP (2000) Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol 40:431–458
Crews FT, Morrow AL, Criswell H, Breese G (1996) Effects of ethanol on ion channels. Int Rev Neurobiol 39:283–367
Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel JA, McIntosh JM, Boulter J, Collins AC, Heinemann SF (2003) The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci 23(35):11045–11053
Damaj MI, Welch SP, Martin BR (1995) In vivo pharmacological effects of dihydro-beta-erythroidine, a nicotinic antagonist, in mice. Psychopharmacology (Berl) 117:67–73
Damaj MI, Glassco W, Dukat M, Martin BR (1999) Pharmacological characterization of nicotine-induced seizures in mice. J Pharmacol Exp Ther 291:1284–1291
David V, Besson M, Changeux JP, Granon S, Cazala P (2006) Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology 50(8):1030–1040
de Fiebre CM, Meyer EL (1995) Ethanol affects the function of nicotinic receptor subtypes expressed in Xenopus oocytes. Alcohol Clin Exp Res 19:Abstract 504
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85(14):5274–5278
Engel JA (1977) Neurochemical aspects of the euphoria induced by dependence-producing drugs. In: Ideström CM (ed) Recent advances in the study of alcoholism (Excerpta Medica International Congress Series 407). Excerpta Medica, Amsterdam, pp 16–22
Engel JA, Blomqvist O, Ericson M, Söderpalm B (1999) Neurochemical and behavioural studies on ethanol and nicotine interactions. In: Palomo T, Beninger RJ, Archer T (eds) Interactive monoaminergic basis of brain disorders. Editorial Sintesis, Madrid, pp 231–248
Ericson M, Blomqvist O, Engel JA et al (1998) Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol 358:189–196
Ericson M, Molander A, Löf E et al (2003) Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol 467:85–93
Gommans J, Stolerman IP, Shoaib M (2000) Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/ 6J mice. Neuropharmacology 39:2840–2847
Grant KA (1994) Emerging neurochemical concepts in the actions of ethanol at ligand-gated ion channels. Behav Pharmacol 5:383–405
Harris RA (1999) Ethanol action on multiple ion channels: which are important? Alcohol Clin Exp Res 23(10):1563–1570
Heidbreder CA, Hagan JJ (2005) Novel pharmacotherapeutic approaches for the treatment of drug addiction and craving. Curr Opin Pharmacol 5(1):107–118
Holladay MW, Dart MJ, Lynch JK (1997) Neuronal nicotinic acetylcholine receptors as targets for drug discovery. J Med Chem 40:4169–4194
Jerlhag E, Grötli M, Luthman K, Svensson L, Engel JA (2006) Role of the subunit composition of central nicotinic acetylcholine receptors for the stimulatory and dopamine-enhancing effects of ethanol in mice. Alcohol Alcohol doi:10.1093/alcalc/agl049
Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21(5):1452–1463
Lança AJ, Adamson KL, Coen KM, Chow BL, Corrigall WA (2000) The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience 96:735–742
Larsson A, Engel JA (2004) Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci Biobehav Rev 27(8):713–720
Larsson A, Svensson L, Söderpalm B, Engel JA (2002) Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol 28:157–167
Larsson A, Jerlhag E, Svensson L, Soderpalm B, Engel JA (2004) Is an alpha-conotoxin MII-sensitive mechanism involved in the neurochemical, stimulatory, and rewarding effects of ethanol? Alcohol 34(2–3):239–250
Larsson A, Edstrom L, Svensson L, Soderpalm B, Engel JA (2005) Voluntary ethanol intake increases extracellular acetylcholine levels in the ventral tegmental area in the rat. Alcohol Alcohol 40(5):349–358
Laviolette SR, van der Kooy D (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5(1):55–65
Lê AD, Corrigall WA, Harding JW, Juzytsch W, Li T-K (2000) Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res 24:155–163
Le Novere N, Corringer PJ, Changeux JP (2002) The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol 53(4):447–456
Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y (2006) Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci 26(6):1872–1879
Löf E (2006) Conditional and non-conditional reward-related responses to alcohol-nicotinic mechanisms. Thesus.Vasastadens Bokbinderi AB, Västra Frölunda ISBN:13:978-91-628-7008-9
Maisonneuve IM, Glick SD (2003) Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol Biochem Behav 75(3):607–618
Mifsud J-C, Hernandez L, Hoebel BG (1989) Nicotine infused into the nucleus accumbens increases synaptic dopamine as measured by in vivo microdialysis. Brain Res 478:365–367
Narahashi T, Aistrup GL, Marszalec W, Nagata K (1999) Neuronal nicotinic acetylcholine receptors: a new target site of ethanol. Neurochem Int 35:131–141
Nisell M, Nomikos GG, Svensson TH (1994) Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol 75:348–352
O’Dell TJ, Christensen BN (1988) Mecamylamine is a selective non-competitive antagonist of N-methyl-D-aspartate- and aspartate induced currents in horizontal cells dissociated from the catfish retina. Neurosci Lett 94:93–98
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. Elsevier Academic Press, Amsterdam
Pickering C, Liljequist S (2003) Cue-induced behavioural activation: a novel model of alcohol craving? Psychopharmacology (Berl) 168:307–313
Pierce RC, Kumaresan V (2006) The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30(2):215–238
Rada PV, Mark GP, Yeomans JJ, Hoebel BG (2000) Acetylcholine release in ventral tegmental area by hypothalamic self-stimulation, eating, and drinking. Pharmacol Biochem Behav 65:375–379
Schilström B, Svensson HM, Svensson TH, Nomikos GG (1998) Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of _7 nicotinic receptors in the ventral tegmental area. Neuroscience 85:1005–1009
Söderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA (2000) Nicotinic mechanisms involved in he dopamine activating and reinforcing properties of ethanol. Behav Brain Res 113:85–96
Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE (2007) Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Nat Am Soc 104:12518–12523
Turek JW, Kang CH, Campbell JE, Arneric SP, Sullivan JP (1995) A sensitive technique for the detection of the _7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J Neurosci Methods 61:113–118
Vailati S, Hanke W, Bejan A, Barabino B, Longhi R, Balestra B, Moretti M, Clementi F, Gotti C (1999) Functional a6-containing nicotinic receptors are present in chick retina. Mol Pharmacol 56:11–19
Watkins SS, Epping-Jordan MP, Koob GF, Markou A (1999) Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav 62(4):743–751
Wise RA, Rompre PP (1989) Brain dopamine and reward. Annu Rev Psychol 40:191–225
Wonnacott S, Albuquerque EX, Bertrand D (1993) Methyllycaconitine: a new probe that discriminates between nicotinic acetylcholine receptor subclasses. Methods Neurosci 12:263–275
Wonnacott S, Sidhpura N, Balfour DJ (2005) Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol 5(1):53–59
Yang X, Criswell HE, Breese GR (1999) Action of ethanol on responses to nicotine from cerebellar interneurons and medial septal neurons: relationship to methyllycaconitine inhibition of nicotine responses. Alcohol Clin Exp Res 23:983–990
Yeomans JS (1995) Role of tegmental cholinergic neurons in dopaminergic activation, antimuscarinic psychosis and schizophrenia. Neuropsychopharmacology 12:3–16
Yoshida K, Engel JA, Liljequist S (1982) The effect of chronic ethanol administration of high affinity 3H-nicotinic binding in rat brain. Naunyn Schmiedebergs Arch Pharmacol 321:74–76
Zanetti L, Picciotto MR, Zoli M (2007) Differential effects of nicotinic antagonists perfused into the nucleus accumbens or the ventral tegmental area on cocaine-induced dopamine release in the nucleus accumbens of mice. Psychopharmacology (Berl) 90(2):189–199
Acknowledgements
This work was supported by grants from the Alcohol Research Council of the Swedish Retailing Monopoly, the Karolinska Institutet, the Swedish Labor Market Insurance (AFA), the Swedish Science Research Council (projects 4247, 7688, 15052), Wilhelm and Martina Lundgrens Scientific Foundation, Rådman and Fru Ernst Collianders Foundation, Stiftelsen Olle Engkvist Byggmästare, Knut and Alice Wallenberg Foundation, The Adlerbertska Foundation, the Filip Lundbergs Foundation, the Längmanska Cultural Foundation, and the Royal Society of Arts and Sciences in Göteborg.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuzmin, A., Jerlhag, E., Liljequist, S. et al. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology 203, 99–108 (2009). https://doi.org/10.1007/s00213-008-1375-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1375-5