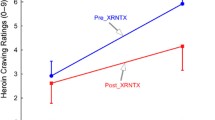
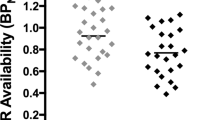
Abstract
Rationale
The perception that smoking relieves negative affect contributes to smoking persistence. Endogenous opioid neurotransmission, and the μ-opioid receptor (MOR) in particular, plays a role in affective regulation and is modulated by nicotine.
Objectives
We examined the relationship of MOR binding availability in the amygdala to the motivation to smoke for negative affect relief and to the acute effects of smoking on affective responses.
Methods
Twenty-two smokers were scanned on two separate occasions after overnight abstinence using [11C]carfentanil positron emission tomography imaging: after smoking a nicotine-containing cigarette and after smoking a denicotinized cigarette. Self-reports of smoking motives were collected at baseline, and measures of positive and negative affect were collected pre- and post- cigarette smoking.
Results
Higher MOR availability in the amygdala was associated with motivation to smoke to relieve negative affect. However, MOR availability was unrelated to changes in affect after smoking either cigarette.
Conclusions
Increased MOR availability in amygdala may underlie the motivation to smoke for negative affective relief. These results are consistent with previous data highlighting the role of MOR neurotransmission in smoking behavior.


Similar content being viewed by others
References
Balerio GN, Aso E, Maldonado R (2005) Involvement of the opioid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology (Berl) 181:260–269
Benowitz NL, Jacob P 3rd, Herrera B (2006) Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clin Pharmacol Ther 80:703–714
Berrendero F, Kieffer BL, Maldonado R (2002) Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci 22:10935–10940
Boyadjieva NI, Sarkar DK (1997) The secretory response of hypothalamic beta-endorphin neurons to acute and chronic nicotine treatments and following nicotine withdrawal. Life Sci 61:PL59–PL66
Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG (2009) Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol 12:305–316
CDC (2009) Center for Disease Control: Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR Morb Mortal Wkly Rep 58:1227–1232
Colvin PJ, Mermelstein RJ (2010) Adolescents' smoking outcome expectancies and acute emotional responses following smoking. Nicotine Tob Res 12:1203–1210
Conklin CA, Perkins KA (2005) Subjective and reinforcing effects of smoking during negative mood induction. J Abnorm Psychol 114:153–164
Costa PT Jr, McCrae RR, Bosse R (1980) Smoking motive factors: a review and replication. Int J Addict 15:537–549
Davidson RJ, Irwin W (1999) The functional neuroanatomy of emotion and affective style. Trends Cogn Sci 3:11–21
Diekhof EK, Geier K, Falkai P, Gruber O (2011) Fear is only as deep as the mind allows a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage 58:275–285
Diener E, Emmons RA (1984) The independence of positive and negative affect. J Pers Soc Psychol 47:1105–1117
Epperson CN, Toll B, Wu R, Amin Z, Czarkowski KA, Jatlow P, Mazure CM, O'Malley SS (2010) Exploring the impact of gender and reproductive status on outcomes in a randomized clinical trial of naltrexone augmentation of nicotine patch. Drug Alcohol Depend 112:1–8
Greenwald M, Johanson CE, Bueller J, Chang Y, Moody DE, Kilbourn M, Koeppe R, Zubieta JK (2007) Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry 61:101–110
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127
Hirvonen J, Aalto S, Hagelberg N, Maksimow A, Ingman K, Oikonen V, Virkkala J, Nagren K, Scheinin H (2009) Measurement of central mu-opioid receptor binding in vivo with PET and [11C]carfentanil: a test–retest study in healthy subjects. Eur J Nucl Med Mol Imaging 36:275–286
Horn D, Waingrow S (1966) Some dimensions of a model for smoking behavior change. Am J Public Health Nations Health 56(Suppl 56):21–26
Houdi AA, Pierzchala K, Marson L, Palkovits M, Van Loon GR (1991) Nicotine-induced alteration in Tyr–Gly–Gly and Met-enkephalin in discrete brain nuclei reflects altered enkephalin neuron activity. Peptides 12:161–166
Hutchison KE, Monti PM, Rohsenow DJ, Swift RM, Colby SM, Gnys M, Niaura RS, Sirota AD (1999) Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacology (Berl) 142:139–143
Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE (2003) Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 23:1096–1112
Juliano LM, Fucito LM, Harrell PT (2011) The influence of nicotine dose and nicotine dose expectancy on the cognitive and subjective effects of cigarette smoking. Exp Clin Psychopharmacol 19:105–115
Karp JS, Surti S, Daube-Witherspoon ME, Freifelder R, Cardi CA, Adam LE, Bilger K, Muehllehner G (2003) Performance of a brain PET camera based on anger-logic gadolinium oxyorthosilicate detectors. J Nucl Med 44:1340–1349
Kassel JD, Evatt DP, Greenstein JE, Wardle MC, Yates MC, Veilleux JC (2007) The acute effects of nicotine on positive and negative affect in adolescent smokers. J Abnorm Psychol 116:543–553
Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ (2011) The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 223:403–410
Lee MR, Gallen CL, Zhang X, Hodgkinson CA, Goldman D, Stein EA, Barr CS (2011) Functional Polymorphism of the Mu-Opioid Receptor Gene (OPRM1) Influences Reinforcement Learning in Humans. PLoS One 6:e24203
Lerman C, Audrain-McGovern J (2010) Reinforcing effects of smoking: more than a feeling. Biol Psychiatry 67:699–701
Lerman C, Audrain J, Orleans CT, Boyd R, Gold K, Main D, Caporaso N (1996) Investigation of mechanisms linking depressed mood to nicotine dependence. Addict Behav 21:9–19
Lerman C, Caporaso N, Main D, Audrain J, Boyd NR, Bowman ED, Shields PG (1998) Depression and self-medication with nicotine: the modifying influence of the dopamine D4 receptor gene. Health Psychol 17:56–62
Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Niaura R, Epstein L (2002) Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend 67:219–223
Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, Shields PG, Kaufmann V, Redden D, Benowitz N, Berrettini WH (2004) The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J 4:184–192
Mague SD, Blendy JA (2010) OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend 108:172–182
Mahler SV, Berridge KC (2009) Which cue to "want?" Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 29:6500–6513
Marty MA, Erwin VG, Cornell K, Zgombick JM (1985) Effects of nicotine on beta-endorphin, alpha MSH, and ACTH secretion by isolated perfused mouse brains and pituitary glands, in vitro. Pharmacol Biochem Behav 22:317–325
Mills RH, Sohn RK, Micevych PE (2004) Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci 24:947–955
Munafo MR, Elliot KM, Murphy MF, Walton RT, Johnstone EC (2007) Association of the mu-opioid receptor gene with smoking cessation. Pharmacogenomics J 7:353–361
O'Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, Blakeslee A, Meandzija B, Romano-Dahlgard D, Wu R, Makuch R, Jatlow P (2006) A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Arch Intern Med 166:667–674
Perkins KA, Giedgowd GE, Karelitz JL, Conklin CA, Parzynski CS (2011) Expectancy for negative affect relief due to smoking may not be predictive under acute mood situations. Exp Clin Psychopharmacol
Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, Sayette MA (2010) Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology (Berl) 210:25–34
Perkins KA, Lerman C, Grottenthaler A, Ciccocioppo MM, Milanak M, Conklin CA, Bergen AW, Benowitz NL (2008) Dopamine and opioid gene variants are associated with increased smoking reward and reinforcement owing to negative mood. Behav Pharmacol 19:641–649
Pessoa L (2010) Emergent processes in cognitive-emotional interactions. Dialogues Clin Neurosci 12:433–448
Pfeiffer A, Pasi A, Mehraein P, Herz A (1982) Opiate receptor binding sites in human brain. Brain Res 248:87–96
Ray R, Jepson C, Patterson F, Strasser A, Rukstalis M, Perkins K, Lynch KG, O'Malley S, Berrettini WH, Lerman C (2006) Association of OPRM1 A118G variant with the relative reinforcing value of nicotine. Psychopharmacology (Berl) 188:355–363
Ray R, Jepson C, Wileyto EP, Dahl JP, Patterson F, Rukstalis M, Pinto A, Berrettini W, Lerman C (2007) Genetic variation in mu-opioid-receptor-interacting proteins and smoking cessation in a nicotine replacement therapy trial. Nicotine Tob Res 9:1237–1241
Ray R, Ruparel K, Newberg A, Wileyto EP, Loughead JW, Divgi C, Blendy JA, Logan J, Zubieta JK, Lerman C (2011) Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc Natl Acad Sci U S A 108:9268–9273
Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK (2005) Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry 29:1264–1280
Rose JE (2006) Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 184:274–285
Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S, Zubieta JK (2007) Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology 32:450–457
Shiffman S (1993) Assessing smoking patterns and motives. J Consult Clin Psychol 61:732–742
Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel JD (1997) Remember that? A comparison of real-time versus retrospective recall of smoking lapses. J Consult Clin Psychol 65:292–300
Shiffman S, Prange M (1988) Self-reported and self-monitored smoking patterns. Addict Behav 13:201–204
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):S208–S219
Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK (2006) Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci 26:5777–5785
Strasser AA, Ashare RL, Kozlowski LT, Pickworth WB (2005a) The effect of filter vent blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol Biochem Behav 82:320–329
Strasser AA, Kaufmann V, Jepson C, Perkins KA, Pickworth WB, Wileyto EP, Rukstalis M, Audrain-McGovern J, Lerman C (2005b) Effects of different nicotine replacement therapies on postcessation psychological responses. Addict Behav 30:9–17
Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA (2007) New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend 86:294–300
Tate JC, Stanton AL (1990) Assessment of the validity of the Reasons for Smoking scale. Addict Behav 15:129–135
Walsh Z, Epstein A, Munisamy G, King A (2008) The impact of depressive symptoms on the efficacy of naltrexone in smoking cessation. J Addict Dis 27:65–72
Walters CL, Cleck JN, Kuo YC, Blendy JA (2005) Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron 46:933–943
Wewers ME, Dhatt RK, Snively TA, Tejwani GA (1999) The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Res 822:107–113
WHO (2009) World Health Organization. WHO Report on the global tobacco epidemic.
Zhang L, Kendler KS, Chen X (2006) The mu-opioid receptor gene and smoking initiation and nicotine dependence. Behav Brain Funct 2:28
Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS (2005) Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 25:7754–7762
Zubieta JK, Dannals RF, Frost JJ (1999) Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry 156:842–848
Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA (2003) Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry 60:1145–1153
Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS (2001) Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293:311–315
Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS (2002) mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci 22:5100–5107
Acknowledgements
We thank the following individuals for their contributions to the study: Dr. Richard Freifelder, Dr. Joel Karp, Dr. Alexander Schmitz, and Rahul Poria for [11C]carfentanil synthesis; Dr. Daniel Pryma and Dr. Rodolfo Perini for serving as PET center injectors; and Dr. Janet Reddin and PET center technologists for PET acquisition and preprocessing at the PET center. This research was supported by National Institute on Drug Abuse Grants R21-DA027066 (to C.L. and J.A.B.) and U01-DA020830 (to C.L.), National Cancer Institute Grant P50-CA143187 (to C.L. and J.A.B.), and a grant from the Pennsylvania Department of Health. C.L. has served as a consultant for and/or received research support from Pfizer, AstraZeneca, Novartis, and GlaxoSmithKline. R.R. has received research support from Pfizer. This research was not supported by industry funds. The authors declare that they have full control of all primary data and they agree to allow the journal to review their data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falcone, M., Gold, A.B., Wileyto, E.P. et al. μ-Opioid receptor availability in the amygdala is associated with smoking for negative affect relief. Psychopharmacology 222, 701–708 (2012). https://doi.org/10.1007/s00213-012-2673-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2673-5