Abstract
Objective
The aims of this study were to evaluate the impact of the CYP2D6 polymorphism on both the steady-state plasma concentrations (Cp) and the clinical outcome of donepezil, a selective acetylcholinesterase inhibitor used in the treatment of Alzheimer’s disease (AD).
Methods
Forty-two patients of Caucasian ethnicity affected by probable AD were included in the study. All had been receiving therapy with donepezil for at least 3 months: 31 patients with 5 mg/day and 11 patients with 10 mg/day. The CYP2D6 genotype was analysed, and donepezil Cp was measured by using high-performance liquid chromatography.
Results
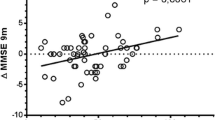
On the basis of their CYP2D6 genotype, 30 patients could be classified as homozygous extensive metabolizers (EM), 10 as heterozygous EM and 2 as ultrarapid metabolizers (UM). No poor metabolizer was found. The dose and body weight-corrected median donepezil Cp were slightly, though not significantly, lower in homozygous than in heterozygous EM (0.33 vs. 0.41 ng/ml/mg/kg, respectively). The latter group consistently showed a better clinical response to treatment, as measured by change in Mini-Mental State Examination score (median: 1.40 vs. −1.30, respectively). UM patients had lower Cp than EM patients and showed no clinical improvement.
Conclusions
Our preliminary data suggest that the CYP2D6 polymorphism influences both donepezil metabolism and therapeutic outcome and that a knowledge of a patient’s CYP2D6 genotype together with donepezil concentration measurements might be useful in the context of improving the clinical efficacy of donepezil therapy.



Similar content being viewed by others
References
Barner EL, Gray SL (1998) Donepezil use in Alzheimer disease. Ann Pharmacother 32:70–77
Broly F, Gaedigk A, Heim M, Eichelbaum M, Morike K, Meyer UA (1991) Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA Cell Biol 10:545–558
Cummings JL (2004) Alzheimer’s disease. N Engl J Med 351:56–67
Dahl M-L, Johansson I, Porsmyr Palmertz M, Ingelman-Sundberg M, Sjöqvist F (1992) Analysis of the CYP2D6 gene in relation to debrisoquine and desipramine hydroxylation in a Swedish population. Clin Pharmacol Ther 51:12–17
Doody RS, Stevens JC, Beck C, Dubinsky RM, Kaye JA, Gwyther L, Mohs RC, Thal LJ, Whitehouse PJ, DeKosky ST, Cummings JL (2001) Practice parameter: management of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56:1154–1166
Dresser GK, Spence JD, Bailey DG (2000) Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet 38:41–57
Hersberger M, Marti-Jaun J, Rentsch K, Hanseler E (2000) Rapid detection of the CYP2D6*3, CYP2D6*4, and CYP2D6*6 alleles by tetra-primer PCR and of the CYP2D6*5 allele by multiplex long PCR. Clin Chem 46:1072–1077
Ingelman-Sundberg M, Oscarson M, McLellan RA (1999) Polymorphic human cytochrome enzymes: an opportunity for individualized drug treatment. Trends Pharmacol Sci 20:342–349
Lovlie R, Daly AK, Molven A, Idle JR, Steen VM (1996) Ultrarapid metabolism of debrisoquine. Characterisation and PCR-based detection of alleles with duplication of the CYP2D6 gene. FEBS Lett 392:30–34
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34:939–944
Meyer UA (2000) Drugs in special patient groups: clinical importance of genomics in drug effects. In: Carruthers GS, Hoffmann BB, Melmon KL, Nieremberg DW (eds) Clinical pharmacology. Basic principles in therapeutics. McGraw Hill, New York, pp 1179–1205
Rogers SL, Friedhoff LT (1998) Pharmacokinetic and pharmacodynamic profile of donepezil HCl following single oral doses. Br J Clin Pharmacol 46[Suppl 1]:1–6
Rogers SL, Cooper NM, Sukovaty R, Pederson JE, Lee JN, Friedhoff LT (1998) Pharmacokinetic and pharmacodynamic profile of donepezil HCl following multiple oral doses. Br J Clin Pharmacol 46[Suppl 1]:7–12
Rogers SL, Doody RS, Mohs RC, Friedhoff LT (1998) Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med 158:1021–1031
Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT (1998) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 50:136–145
Saitoh T, Xia Y, Chen X, Masliah E, Galasko D, Shults C, Thal LJ, Hansen LA, Katzman R (1995) The CYP2D6B mutant allele is overrepresented in the Lewy body variant of Alzheimer’s disease. Ann Neurol 37:110–112
Scordo MG, Caputi AP, D’Arrigo C, Fava G, Spina E (2004) Allele and genotype frequencies of CYP2C9, CYP2C19 and CYP2D6 in an Italian population. Pharmacol Res 50:195–200
Scordo MG, Dahl M-L, Spina E, Cordici F, Arena MG (2006) No association between CYP2D6 polymorphism and Alzheimer’s disease in an Italian population. Pharmacol Res 53:162–165
Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, Finkel SI, Gwyther LP, Khachaturian ZS, Lebowitz BD, McRae TD, Morris JC, Oakley F, Schneider LS, Streim JE, Sunderland T, Teri LA, Tune LE (1997) Diagnosis and treatment of Alzheimer’s disease and related disorders: consensus statement of the American Association of Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA 278:1363–1371
Smith CAD, Gough AC, Leigh PN, Summers BA, Harding AE, Maraganore DM, Sturman SG, Schapira AHV, Williams AC, Spurr NK, Wolf CR (1992) Debrisoquine hydroxylase gene polymorphism and susceptibility to Parkinson’s disease. Lancet 339:1375–1377
Steijns LS, Van Der Weide J (1998) Ultrarapid drug metabolism: PCR-based detection of CYP2D6 gene duplication. Clin Chem 44:914–917
Taniguchi R, Kumai T, Matsumoto N, Watanabe M, Kamio K, Suzuki S, Kobayashi S (2005) Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci 97:83–90
Tiseo PJ, Perdomo CA, Friedhoff LT (1998a) Concurrent administration of donepezil HCl and cimetidine: assessment of pharmacokinetic changes following single and multiple doses. Br J Clin Pharmacol 46(Suppl 1):25–29
Tiseo PJ, Perdomo CA, Friedhoff LT (1998b) Concurrent administration of donepezil HCl and ketoconazole: assessment of pharmacokinetic changes following single and multiple doses. Br J Clin Pharmacol 46[Suppl 1]:30–34
Tiseo PJ, Perdomo CA, Friedhoff LT (1998c) Metabolism and elimination of 14C-donepezil in healthy volunteers: a single-dose study. Br J Clin Pharmacol 46[Suppl 1]:19–24
Whitehead A, Perdomo C, Pratt RD, Birks J, Wilcock GK, Evans JG (2004) Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer’s disease: a metaanalysis of individual patient data from randomised controlled trials. Int J Geriatr Psychiatry 19:624–633
Yamada H, Dahl M-L, Viitanen M, Winblad B, Sjoqvist F, Lannfelt L (1998) No association between familial Alzheimer disease and cytochrome P450 polymorphisms. Alzheimer Dis Assoc Disord 12:204–207
Yasui-Furukori N, Furuya R, Takahata T, Tateishi T (2002) Determination of donepezil, an acetylcholinesterase inhibitor, in human plasma by high-performance liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Anal Technol Biomed Life Sci 768:261–265
Zhou S, Chan E, Lim LY, Boelsterli UA, Li SC, Wang J, Zhang Q, Huang M, Xu A (2004) Therapeutic drugs that behave as mechanism-based inhibitors of cytochrome P450 3A4. Curr Drug Metab 5:415–442
Acknowledgements
This study was supported by the Piedmont County Grants for Research (Turin, Italy), by grants from SIF (“Società Italiana di Farmacologia”), Pfizer Italia Srl and Pfizer AB (Sweden), the Swedish Medical Society and the Swedish Research Council (2002–6475). We thank Dr. Norio Yasui-Furukori (Hirosaki University, School of Medicine, Japan) for the generous gift of donepezil, and Prof. Maurizio Rinaldi and Dr. R. Gindro (DISCAFF Department, University of Eastern Piedmont, Italy) for advice on statistical analysis. The study protocol was approved by the Research Ethics Committee at the “Ospedale Maggiore della Carità” of Novara (Italy), in accordance with the ethical standards laid down in the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varsaldi, F., Miglio, G., Scordo, M.G. et al. Impact of the CYP2D6 polymorphism on steady-state plasma concentrations and clinical outcome of donepezil in Alzheimer’s disease patients. Eur J Clin Pharmacol 62, 721–726 (2006). https://doi.org/10.1007/s00228-006-0168-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0168-1