Abstract
Objectives
To assess the comparative pharmacokinetic profile and bioavailability of docosahexaenoic acid (DHA)/eicosapentaenoic acid (EPA) after multiple-dose administration of a new oral formulation (test formulation) and a commercially available reference formulation in healthy subjects.
Methods
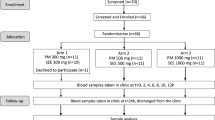
Forty-eight healthy subjects received a 28-day oral treatment with DHA/EPA in the form of either the test or the reference product according to an open-label, randomized, parallel-group design. Both formulations were given t.i.d. at 8-h intervals at a dose of 3.0 g/day. Steady-state DHA and EPA concentrations in plasma and lysed whole blood were measured by gas-liquid chromatography at baseline and after 7, 14, 21 and 28 days of treatment. Kinetic parameters were compared both after subtraction of baseline concentrations and by using baseline concentrations as a covariate.
Results
For both DHA and EPA, plasma and RBC concentrations measured from day 7 to day 28 were significantly higher than at baseline and did not differ significantly between the two products. On day 28 the plasma DHA concentration on average doubled the baseline level after administration of test and reference product, while there was a 10-fold increase in EPA plasma concentration. When the assessment was performed using baseline values as covariate, test-to-reference ratios for area under the curve (AUCss0–8) and for peak concentration (Cssmax) after the last administration on day 28 met bioequivalence criteria (i.e., 90% confidence intervals within 0.80–1.25 for AUCss0–8 ratios, and within 0.75–1.33 for Cssmax ratios). When the assessment was conducted after subtraction of baseline values, the 90% confidence intervals for Cssmax ratios were within the bioequivalence range, whereas the intervals for AUCss0–8 ratio were borderline for bioequivalence.
Conclusion
The two formulations tested were similarly effective in increasing DHA and EPA concentrations in plasma and lysed whole blood, and showed comparable bioavailability for both active components.



Similar content being viewed by others
References
Piscione F, Pignalberi C, Totteri A, Messina F, Altamura G (2007) Cardiovascular effects of omega-3 free fatty acids. Curr Vasc Pharmacol 5(2):163–172
Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE Jr, Marangell LB, Richardson AJ, Lake J, Stoll AL (2006) Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 67(12):1954–1967
Lee S, Gura KM, Kim S, Arsenault DA, Bistrian BR, Puder M (2006) Current clinical applications of omega-6 and omega-3 fatty acids. Nutr Clin Pract 21(4):323–341
Weber HS, Selimi D, Huber G (2006) Prevention of cardiovascular diseases and highly concentrated n-3 polyunsaturated fatty acids (PUFAs). Herz 31(Suppl 3):24–30
Ruxton CH, Reed SC, Simpson MJ, Millington KJ (2007) The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 20(3):275–285
von Schacky C (2007) Omega-3 fatty acids and cardiovascular disease. Curr Opin Clin Nutr Metab Care 10(2):129–135
He K, Rimm EB, Merchant A et al (2002) Fish consumption and risk of stroke in men. JAMA 288(24):3130–3135
Burr ML, Fehily AM, Gilbert JF et al (1989) Effects of changes in fat, fish, and fibres intake on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 2:757–761
Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 354:47–55
Gibney MJ, Daly E (1994) The incorporation of n-3 polyunsaturated fatty acids into plasma lipid and lipoprotein fractions in the postprandial phase in healthy volunteers. Eur J Clin Nutr 48:866–872
Hodge J, Sanders K, Sinclair AJ (1993) Differential utilization of eicosapentaenoic acid and docosahexaenoic acid in human plasma. Lipids 28(6):525–531
Marsen TA, Pollok M, Oette K et al (1992) Pharmacokinetics of omega-3-fatty acids during ingestion of fish oil preparations. Prostaglandins Leukot Essent Fat Acids 46(3):191–196
Marangoni F, Angeli MT, Colli S et al (1993) Changes of n-3 and n-6 fatty acids in plasma and circulating cells of normal subjects, after prolonged administration of 20:5 (EPA) and 22:6 (DHA) ethyl esters and prolonged washout. Biochim Biophys Acta 1210(1):55–62
Vidgren HM, Agren JJ, Schwab U, Rissanen T, Hänninen O, Uusitupa MI (1997) Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids 32(7):697–705
Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY (2006) Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem 52(12):2265–2272
Bruno MJ, Koeppe RE, Andersen OS (2007) Docosahexaenoic acid alters bilayer elastic properties. Proc Natl Acad Sci USA 104(23):9638–9643
Lundbaek JA, Andersen OS (1994) Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J Gen Physiol 104(4):645–673
Lund EK, Harvey LJ, Ladha S, Clark DC, Johnson IT (1999) Effects of dietary fish oil supplementation on the phospholipid composition and fluidity of cell membranes from human volunteers. Ann Nutr Metab 43(5):290–300
Arterburn LM, Hall EB, Oken H (2006) Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr 83(6 Suppl):1467S–1476S
Department of Health and Human Services and U.S. Department of Agriculture (2005) Dietary guidelines for Americans. http://www.health.gov/DietaryGuidelines/dga2005/document/default.htm. Accessed 18 Dec 2008
Schuirmann DJ (1990) Design of bioavailability/bioequivalence studies. Drug Inf J 24:315–323
The European Agency for the Evaluation of Medicinal Products (EMEA) (2001) Note for guidance on the investigation of bioavailability and bioequivalence. Guideline CPMP/EWP/QWP/1401/98. http://www.emea.europa.eu/pdfs/human/qwp/140198en.pdf. Accessed 18 Dec 2008
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2003) Guidance for industry—bioavailability and bioequivalence studies for orally administered drug products. General considerations. http://www.fda.gov/Cder/guidance/5356fnl.htm. Accessed 18 Dec 2008
Food and Drug Administration, Advisory Committee for Pharmaceutical Science (2004) Briefing information: bioequivalence requirements for highly variable drugs and drug products. Washington DC
Tothfalusi L, Endrenyi L, Midha KK et al (2001) Evaluation of the bioequivalence of highly variable drugs and drug products. Pharm Res 18:728–733
Tothfalusi L, Endrenyi L (2003) Limits for the scaled average bioequivalence of highly variable drugs and drug products. Pharm Res 20:382–389
Tothfalusi L, Endrenyi L, Midha KK (2003) Scaling or wider bioequivalence limits for highly variable drugs and for the special case of Cmax. Int J Clin Pharmacol Ther 41:217–225
Marzo A (1999) Open questions on bioequivalence: some problems and some solutions. Pharmacol Res 40(4):357–368
SAS/STAT user’s guide, version 8.2
Thermo Electron (2005) Kinetica 4.4.1 user’s manual. Waltham, MA
Blonk MC, Bilo HJ, Nauta JJ, Popp-Snijders C, Mulder C, Donker AJ (1990) Dose-response effects of fish-oil supplementation in healthy volunteers. Am J Clin Nutr 52(1):120–127
Acknowledgements
IBSA Institut Biochimique SA, Switzerland, provided the study medication. The relationships between the sponsor and the institutions of AR, ADS and MVD were regulated by financial agreements. CS is a full-time employee of IBSA. EP has received speaker’s or consultancy fees or research/travel grants from Cyberonics, GSK, Janssen-Cilag, Johnson & Johnson, IBSA, Novartis, Pfizer, Sanofi-Aventis, Schwartz-Pharma, SK Holdings, UCB Pharma, Valeant. All experimental procedures performed in the study were in accordance with Swiss law. No potential conflict of interest exists in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rusca, A., Di Stefano, A.F.D., Doig, M.V. et al. Relative bioavailability and pharmacokinetics of two oral formulations of docosahexaenoic acid/eicosapentaenoic acid after multiple-dose administration in healthy volunteers. Eur J Clin Pharmacol 65, 503–510 (2009). https://doi.org/10.1007/s00228-008-0605-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0605-4