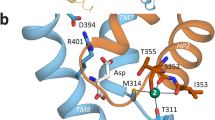
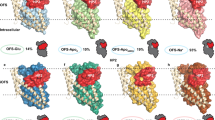
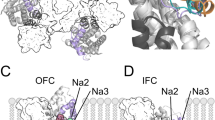
Abstract
Neurotransmitter transporters are key elements in the termination of the synaptic actions of the neurotransmitters. They use the energy stored in the electrochemical ion gradients across the plasma membrane of neurons and glial cells for uphill transport of the transmitters into the cells surrounding the synapse. Therefore specific transporter inhibitors can potentially be used as novel drugs for neurological disease. Sodium-coupled neurotransmitter transporters belong to either of two distinct families. The glutamate transporters belong to the SLC1 family, whereas the transporters of the other neurotransmitters belong to the SLC6 family. An exciting and recent development is the emergence of the first high-resolution structures of archeal and bacterial members belonging to these two families. In this review the functional results on prototypes of the two families, the GABA transporter GAT-1 and the glutamate transporters GLT-1 and EAAC1, are described and discussed within the perspective provided by the novel structures.


Similar content being viewed by others
References
Arriza J.L., Eliasof S., Kavanaugh M.P., Amara S.G. 1997. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc. Natl. Acad. Sci. USA 94:4155–4160
Arriza J.L., Fairman W.A., Wadiche J.I., Murdoch G.H., Kavanaugh M.P., Amara S.G. 1994. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 14:5559–5569
Bendahan A., Armon A., Madani N., Kavanaugh M.P., Kanner B.I. 2000. Arginine 447 plays a pivotal role in substrate interactions in a neuronal glutamate transporter. J. Biol. Chem. 275:37436–37442
Bennett E.R., Su H., Kanner B.I. 2000. Mutation of arginine 44 of GAT-1, a (Na(+) + Cl(−))-coupled gamma-aminobutyric acid transporter from rat brain, impairs net flux but not exchange. J. Biol. Chem. 275:34106–34113
Billups B., Rossi D., Attwell D. 1996. Anion conductance behavior of the glutamate uptake carrier in salamander retinal glial cells. J. Neurosci. 16:6722–6731
Bismuth Y., Kavanaugh M.P., Kanner B.I. 1997. Tyrosine 140 of the gammaaminobutyric acid transporter GAT-1 plays a critical role in neurotransmitter recognition. J. Biol. Chem. 272:16096–16102
Borre L., Kanner B.I. 2001. Coupled, but not uncoupled, fluxes in a neuronal glutamate transporter can be activated by lithium ions. J. Biol. Chem. 276:40396–40401
Borre L., Kanner B.I. 2004. Arginine 445 controls the coupling between glutamate and cations in the neuronal transporter EAAC-1. J. Biol. Chem. 279:2513–2519
Borre L., Kavanaugh M.P., Kanner B.I. 2002. Dynamic equilibrium between coupled and uncoupled modes of a neuronal glutamate transporter. J. Biol. Chem. 277:13501–13507
Brew H., Attwell D. 1987. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature 327:707–709
Brocke L., Bendahan A., Grunewald M., Kanner B.I. 2002. Proximity of two oppositely oriented re-entrant loops in the glutamate transporter GLT-1 identified by paired cysteine mutagenesis. J. Biol. Chem. 277:3985–3992
Chen J.G., Liu-Chen S., Rudnick G. 1998. Determination of external loop topology in the serotonin transporter by site-directed chemical labeling. J. Biol. Chem. 273:12675–12681
Eliasof S., Jahr C.E. 1996. Retinal glial cell glutamate transporter is coupled to an anionic conductance. Proc. Natl. Acad. Sci. USA 93:4153–4158
Fairman W.A., Vandenberg R.J., Arriza J.L., Kavanaugh M.P., Amara S.G. 1995. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature 375:599–603
Giros B., Jaber M., Jones S.R., Wightman R.M., Caron M.G. 1996. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379:606–612
Golovanevsky V., Kanner B.I. 1999. The reactivity of the gamma-aminobutyric acid transporter GAT-1 toward sulfhydryl reagents is conformationally sensitive. Identification of a major target residue. J. Biol. Chem. 274:23020–23026
Grewer C., Watzke N., Rauen T., Bicho A. 2003. Is the glutamate residue Glu-373 the proton acceptor of the excitatory amino acid carrier 1? J. Biol. Chem. 278:2585–92
Grunewald M., Bendahan A., Kanner B.I. 1998. Biotinylation of single cysteine mutants of the glutamate transporter GLT-1 from rat brain reveals its unusual topology. Neuron. 21:623–632
Grunewald M., Kanner B. 1995. Conformational changes monitored on the glutamate transporter GLT-1 indicate the existence of two neurotransmitter-bound states. J. Biol. Chem. 270:17017–17024
Grunewald M., Kanner B.I. 2000. The accessibility of a novel re-entrant loop of the glutamate transporter GLT-1 is restricted by its substrate. J. Biol. Chem. 275:9684–9689
Grunewald M., Menaker D., Kanner B.I. 2002. Cysteine-scanning mutagenesis reveals a conformationally sensitive re-entrant pore-loop in the glutamate transporter GLT-1. J. Biol. Chem. 277:26074–26080
Guastella J., Nelson N., Nelson H., Czyzyk L., Keynan S., Miedel M.C., Davidson N., Lester H.A., Kanner B.I. 1990. Cloning and expression of a rat brain GABA transporter. Science 249:1303–1306
Hilgemann D.W., Lu C.C. 1999. GAT1 (GABA:Na+:Cl−) cotransport function. Database reconstruction with an alternating access model. J. Gen. Physiol. 114:459–475
Jiang Y., Lee A., Chen J., Cadene M., Chait B.T., MacKinnon R. 2002. The open pore conformation of potassium channels. Nature 417:523–526
Kanai Y., Hediger M.A. 1992. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature 360:467–471
Kanner B.I. 1978. Active transport of gamma-aminobutyric acid by membrane vesicles isolated from rat brain. Biochemistry 17:1207–1211
Kanner B.I. 1983. Bioenergetics of neurotransmitter transport. Biochim. Biophys. Acta. 726:293–316
Kanner B.I. 1989. Ion-coupled neurotransmitter transport. Curr. Opin. Cell Biol. 1:735–738
Kanner B.I. 2003. Transmembrane domain I of the gamma-aminobutyric acid transporter GAT-1 plays a crucial role in the transition between cation leak and transport modes. J. Biol. Chem. 278:3705–3712
Kanner B.I. 2005. Molecular physiology: intimate contact enables transport. Nature 437:203–205
Kanner B.I., Bendahan A. 1982. Binding order of substrates to the sodium and potassium ion coupled L-glutamic acid transporter from rat brain. Biochemistry 21:6327–6330
Kanner B.I., Borre L. 2002. The dual-function glutamate transporters: structure and molecular characterisation of the substrate-binding sites. Biochim. Biophys. Acta. 1555:92–95
Kanner B.I., Marva E. 1982. Efflux of L-glutamate by synaptic plasma membrane vesicles isolated from rat brain. Biochemistry 21:3143–3147
Kanner B.I., Schuldiner S. 1987. Mechanism of transport and storage of neurotransmitters. CRC Crit. Rev. Biochem. 22:1–38
Kanner B.I., Sharon I. 1978. Active transport of L-glutamate by membrane vesicles isolated from rat brain. Biochemistry 17:3949–3953
Kavanaugh M.P., Arriza J.L., North R.A., Amara S.G. 1992. Electrogenic uptake of gamma-aminobutyric acid by a cloned transporter expressed in Xenopus oocytes. J. Biol. Chem. 267:22007–22009
Kavanaugh M.P., Bendahan A., Zerangue N., Zhang Y., Kanner B.I. 1997. Mutation of an amino acid residue influencing potassium coupling in the glutamate transporter GLT-1 induces obligate exchange. J. Biol. Chem. 272:1703–1708
Keynan S., Kanner B.I. 1988. gamma-Aminobutyric acid transport in reconstituted preparations from rat brain: coupled sodium and chloride fluxes. Biochemistry 27:12–17
Krause S., Schwarz W. 2005. Indentification and selective inhibition of the channel mode of the neuronal GABA transporter 1. Mol. Pharmacol. 68:1728–1735
Levy L.M., Warr O., Attwell D. 1998. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J. Neurosci. 18:9620–9628
Loo D.D., Eskandari S., Boorer K.J., Sarkar H.K., Wright E.M. 2000. Role of Cl− in electrogenic Na+-coupled cotransporters GAT1 and SGLT1. J. Biol. Chem. 275:37414–37422
Lu C.C., Hilgemann D.W. 1999a. GAT1 (GABA:Na+:Cl−) cotransport function. Kinetic studies in giant Xenopus oocyte membrane patches. J. Gen. Physiol. 114:445–457
Lu C.C., Hilgemann D.W. 1999b. GAT1 (GABA:Na+:Cl−) cotransport function. Steady state studies in giant Xenopus oocyte membrane patches. J. Gen. Physiol. 114:429–444
Mabjeesh N.J., Kanner B.I. 1993. The substrates of a sodium- and chloride-coupled gamma-aminobutyric acid transporter protect multiple sites throughout the protein against proteolytic cleavage. Biochemistry 32:8540–8546
MacAulay N., Bendahan A., Loland C.J., Zeuthen T., Kanner B.I., Gether U. 2001. Engineered Zn(2+) switches in the gamma-aminobutyric acid (GABA) transporter-1. Differential effects on GABA uptake and currents. J. Biol. Chem. 276:40476–40485
MacAulay N., Zeuthen T., Gether U. 2002. Conformational basis for the Li(+) induced leak current in the rat gamma-aminobutyric acid (GABA) transporter J. Physiol. 544:447–458
Mager S., Kleinberger-Doron N., Keshet G.I., Davidson N., Kanner B.I., Lester H.A. 1996. Ion binding and permeation at the GABA transporter GAT1. J. Neurosci. 16:5405–5414
Mager S., Min C., Henry D.J., Chavkin C., Hoffman B.J., Davidson N., Lester H.A. 1994. Conducting states of a mammalian serotonin transporter. Neuron 12:845–859
Mager S., Naeve J., Quick M., Labarca C., Davidson N., Lester H.A. 1993. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron 10:177–188
Melamed N., Kanner B.I. 2004. Transmembrane domains I and II of the gamma aminobutyric acid transporter GAT-4 contain molecular determinants of substrate specificity. Mol. Pharmacol. 65:1452–1461
Menaker D., Bendahan A., Kanner B.I. 2006. The substrate specificity of a neuronal glutamate transporter is determined by the nature of the coupling ion. J. Neurochem. 99:20–28
Nelson N. 1998. The family of Na+/Cl− neurotransmitter transporters. J. Neurochem. 71:1785–1803
Nelson P.J., Rudnick G. 1979. Coupling between platelet 5-hydroxytryptamine and potassium transport. J. Biol. Chem. 254:10084–10089
Ogawa H., Toyoshima C. 2002. Homology modeling of the cation binding sites of Na+K+-ATPase. Proc. Natl. Acad. Sci. USA 99:15977–15982
Pantanowitz S., Bendahan A., Kanner B.I. 1993. Only one of the charged amino acids located in the transmembrane alpha-helices of the gamma-aminobutyric acid transporter (subtype A) is essential for its activity. J. Biol. Chem. 268:32225
Pines G., Danbolt N.C., Bjoras M., Zhang Y., Bendahan A., Eide L., Koepsell H., Storm-Mathisen J., Seeberg E., Kanner B.I. 1992. Cloning and expression of a rat brain L-glutamate transporter. Nature 360:464–467
Pines G., Kanner B.I. 1990. Counterflow of L-glutamate in plasma membrane vesicles and reconstituted preparations from rat brain. Biochemistry 29:1120914
Radian R., Bendahan A., Kanner B.I. 1986. Purification and identification of the functional sodium- and chloride-coupled gamma-aminobutyric acid transport glycoprotein from rat brain. J. Biol. Chem. 261:15437–15441
Rosental N., Bendahan A., Kanner B.I. 2006. Multiple consequences of mutating two conserved Beta-bridge forming residues in the translocation cycle of a neuronal glutamate transporter. J. Biol. Chem. 281:27905–27918
Ryan R.M., Mitrovic A.D., Vandenberg R.J. 2004. The chloride permeation pathway of a glutamate transporter and its proximity to the glutamate translocation pathway. J. Biol. Chem. 279:20742–20751
Ryan R.M., Vandenberg R.J. 2002. Distinct conformational states mediate the transport and anion channel properties of the glutamate transporter EAAT-1. J. Biol. Chem. 277:13494–13500
Seal R.P., Shigeri Y., Eliasof S., Leighton B.H., Amara S.G. 2001. Sulfhydryl modification of V449C in the glutamate transporter EAAT1 abolishes substrate transport but not the substrate-gated anion conductance. Proc. Natl. Acad. Sci. USA 98:15324–15329
Shachnai L., Shimamoto K., Kanner B.I. 2005. Sulfhydryl modification of cysteine mutants of a neuronal glutamate transporter reveals an inverse relationship between sodium dependent conformational changes and the glutamate-gated anion conductance. Neuropharmacology 49:862–871
Slotboom D.J., Sobczak I., Konings W.N., Lolkema J.S. 1999. A conserved serine-rich stretch in the glutamate transporter family forms a substrate-sensitive re-entrant loop. Proc. Natl. Acad. Sci. USA 96:14282–14287
Storck T., Schulte S., Hofmann K., Stoffel W. 1992. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc. Natl. Acad. Sci. USA 89:10955–10959
Szatkowski M., Barbour B., Attwell D. 1990. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature 348:443–446
Tanaka K., Watase K., Manabe T., Yamada K., Watanabe M., Takahashi K., Iwama H., Nishikawa T., Ichihara N., Kikuchi T., Okuyama S., Kawashima N., Hori S., Takimoto M., Wada K. 1997. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276:1699–16702
Tao Z., Zhang Z., Grewer C. 2006. Neutralization of the aspartic acid residue Asp 367, but not Asp-454, inhibits binding of Na+ to the glutamate-free form and cycling of the glutamate transporter EAAC1. J. Biol. Chem. 281:10263–10272
Toyoshima C., Nakasako M., Nomura H., Ogawa H. 2000. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 405:647–655
Wadiche J.I., Amara S.G., Kavanaugh M.P. 1995a. Ion fluxes associated with excitatory amino acid transport. Neuron 15:721–728
Wadiche J.I., Arriza J.L., Amara S.G., Kavanaugh M.P. 1995b. Kinetics of a human glutamate transporter. Neuron 14:1019–1027
Weinglass A.B., Smirnova I.N., Kaback H.R. 2001. Engineering conformational flexibility in the lactose permease of Escherichia coli: use of glycine-scanning mutagenesis to rescue mutant Glu325 → Asp. Biochemistry 40:769–776
Yamashita A., Singh S.K., Kawate T., Jin Y., Gouaux E. 2005. Crystal structure of a bacterial homologue of Na+/Cl−dependent neurotransmitter transporters. Nature 437:215–223
Yernool D., Boudker O., Jin Y., Gouaux E. 2004. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature 431:811–818
Zarbiv R., Grunewald M., Kavanaugh M.P., Kanner B.I. 1998. Cysteine scanning of the surroundings of an alkali-ion binding site of the glutamate transporter GLT-1 reveals a conformationally sensitive residue. J. Biol. Chem. 273:14231–14237
Zerangue N., Kavanaugh M.P. 1996. Flux coupling in a neuronal glutamate transporter. Nature 383:634–637
Zhang Y., Bendahan A., Zarbiv R., Kavanaugh M.P., Kanner B.I. 1998. Molecular determinant of ion selectivity of a (Na+ + K+)-coupled rat brain glutamate transporter. Proc. Natl. Acad. Sci. USA 95:751–755
Zhang Y., Kanner B.I. 1999. Two serine residues of the glutamate transporter GLT-1 are crucial for coupling the fluxes of sodium and the neurotransmitter. Proc. Natl. Acad. Sci. USA 96:1710–1715
Zhou Y., Bennett E.R., Kanner B.I. 2004. The aqueous accessibility in the external half of transmembrane domain I of the GABA transporter GAT-1 is modulated by its ligands. J. Biol. Chem. 279:13800–13808
Zhou Y., Kanner B.I. 2005. Transporter-associated currents in the gamma aminobutyric acid transporter GAT-1 are conditionally impaired by mutations of a conserved glycine residue. J. Biol. Chem. 280:20316–20324
Zhou Y., Zomot E., Kanner B.I. 2006. Identification of a lithium interaction site in the GABA transporter GAT-1. J. Biol. Chem. 281:22092–22099
Zomot E., Kanner B.I. 2003. The interaction of the gamma-aminobutyric acid transporter GAT-1 with the neurotransmitter is selectively impaired by sulfhydryl modification of a conformationally sensitive cysteine residue engineered into extracellular loop IV. J. Biol. Chem. 278:42950–42958
Zomot E., Zhou Y., Kanner B.I. 2005. Proximity of transmembrane domains 1 and 3 of the gamma-aminobutyric acid transporter GAT-1 inferred from paired cysteine mutagenesis. J. Biol. Chem. 280:25512–25516
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanner, B.I. Structure and Function of Sodium-coupled GABA and Glutamate Transporters. J Membrane Biol 213, 89–100 (2006). https://doi.org/10.1007/s00232-006-0877-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-006-0877-5