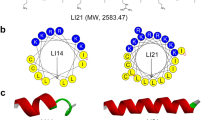
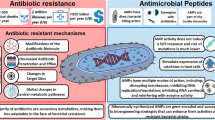
Abstract
Multidrug antibiotic resistance is an increasingly serious public health problem worldwide. Thus, there is a significant and urgent need for the development of new classes of antibiotics that do not induce resistance. To develop such antimicrobial compounds, we must look toward agents with novel mechanisms of action. Membrane-permeabilizing antimicrobial peptides (AMPs) are good candidates because they act without high specificity toward a protein target, which reduces the likelihood of induced resistance. Understanding the mechanism of membrane permeabilization is crucial for the development of AMPs into useful antimicrobial agents. Various models, some phenomenological and others more quantitative or semimolecular, have been proposed to explain the action of AMPs. While these models explain many aspects of AMP action, none of the models captures all of the experimental observations, and significant questions remain unanswered. Here, we discuss the state of the field and pose some questions that, if answered, could speed the discovery of clinically useful peptide antibiotics.

Similar content being viewed by others
References
Almeida PF, Pokorny A (2009) Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry 48:8083–8093
Arias CA, Murray BE (2009) Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N Engl J Med 360:439–443
Bechinger B (2009) Rationalizing the membrane interactions of cationic amphipathic antimicrobial peptides by their molecular shape. Curr Opin Colloid Interface Sci 14:349–355
Bechinger B, Lohner K (2006) Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta 1758:1529–1539
Brahmachary M et al (2004) ANTIMIC: a database of antimicrobial sequences. Nucleic Acids Res 32(database issue D586-9):D586–D589
Cudic M, Otvos L Jr (2002) Intracellular targets of antibacterial peptides. Curr Drug Targets 3:101–106
Demegen Pharmaceuticals (2010) Pharmaceutical products. Product profiles. Novel candidiasis therapy. www.demegen.com/pharma/candidiasis.htm
Easton DM et al (2009) Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol 27:582–590
Epand RM, Epand RF (2009) Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim Biophys Acta 1788:289–294
Epand RF et al (2010) Probing the “charge cluster mechanism” in amphipathic helical cationic antimicrobial peptides. Biochemistry 49:4076–4084
Fjell CD, Hancock RE, Cherkasov A (2007) AMPer: a database and an automated discovery tool for antimicrobial peptides. Bioinformatics 23:1148–1155
Gardy JL et al (2009) Enabling a systems biology approach to immunology: focus on innate immunity. Trends Immunol 30:249–262
Gazit E et al (1996) Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J Mol Biol 258:860–870
Gregory SM et al (2008) A quantitative model for the all-or-none permeabilization of phospholipid vesicles by the antimicrobial peptide cecropin A. Biophys J 94:1667–1680
Gregory SM, Pokorny A, Almeida PF (2009) Magainin 2 revisited: a test of the quantitative model for the all-or-none permeabilization of phospholipid vesicles. Biophys J 96:116–131
Hamill P et al (2008) Novel anti-infectives: is host defence the answer? Curr Opin Biotechnol 19:628–636
Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557
He K et al (1996) Mechanism of alamethicin insertion into lipid bilayers. Biophys J 71:2669–2679
Hong RW et al (2003) Transcriptional profile of the Escherichia coli response to the antimicrobial insect peptide cecropin A. Antimicrob Agents Chemother 47:1–6
Hristova K, Selsted ME, White SH (1997) Critical role of lipid composition in membrane permeabilization by rabbit neutrophil defensins. J Biol Chem 272:24224–24233
Huang HW, Chen FY, Lee MT (2004) Molecular mechanism of peptide-induced pores in membranes. Phys Rev Lett 92:198304
Kazemzadeh-Narbat M et al (2010) Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 31:9519–9526
Klein E, Smith DL, Laxminarayan R (2007) Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis 13:1840–1846
Ladokhin AS, Selsted ME, White SH (1997) Sizing membrane pores in lipid vesicles by leakage of co-encapsulated markers: pore formation by melittin. Biophys J 72:1762–1766
Ludtke SJ et al (1996) Membrane pores induced by magainin. Biochemistry 35:13723–13728
Matsuzaki K, Yoneyama S, Miyajima K (1997) Pore formation and translocation of melittin. Biophys J 73:831–838
Melo MN, Dugourd D, Castanho MA (2006) Omiganan pentahydrochloride in the front line of clinical applications of antimicrobial peptides. Recent Pat Antiinfect Drug Discov 1:201–207
Merrifield RB, Vizioli LD, Boman HG (1982) Synthesis of the antibacterial peptide cecropin A (1–33). Biochemistry 21:5020–5031
Ostolaza H et al (1993) Release of lipid vesicle contents by the bacterial protein toxin α-haemolysin. Biochim Biophys Acta 1147:81–88
Otvos L Jr (2005) Antibacterial peptides and proteins with multiple cellular targets. J Pept Sci 11:697–706
Otvos L Jr et al (2006) Prior antibacterial peptide-mediated inhibition of protein folding in bacteria mutes resistance enzymes. Antimicrob Agents Chemother 50:3146–3149
Pittet D et al (2008) Infection control as a major World Health Organization priority for developing countries. J Hosp Infect 68:285–292
Pokorny A, Almeida PF (2004) Kinetics of dye efflux and lipid flip-flop induced by delta-lysin in phosphatidylcholine vesicles and the mechanism of graded release by amphipathic, alpha-helical peptides. Biochemistry 43:8846–8857
Pokorny A et al (2008) The activity of the amphipathic peptide delta-lysin correlates with phospholipid acyl chain structure and bilayer elastic properties. Biophys J 95:4748–4755
Qian S et al (2008) Structure of the alamethicin pore reconstructed by X-ray diffraction analysis. Biophys J 94:3512–3522
Rapaport D, Shai Y (1991) Interaction of fluorescently labeled pardaxin and its analogues with lipid bilayers. J Biol Chem 266:23769–23775
Rathinakumar R, Wimley WC (2008) Biomolecular engineering by combinatorial design and high-throughput screening: small, soluble peptides that permeabilize membranes. J Am Chem Soc 130:9849–9858
Rathinakumar R, Wimley WC (2010) High-throughput discovery of broad-spectrum peptide antibiotics. FASEB J 24:3232–3238
Rathinakumar R, Walkenhorst WF, Wimley WC (2009) Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: the importance of interfacial activity. J Am Chem Soc 131:7609–7617
Sengupta D et al (2008) Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim Biophys Acta 1778:2308–2317
Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Biopolymers 66:236–248
Steiner H et al (1981) Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292:246–248
Wang Z, Wang G (2004) APD: the antimicrobial peptide database. Nucleic Acids Res 32:590–592
Westerhoff HV et al (1989) Magainins and the disruption of membrane-linked free-energy transduction. Proc Natl Acad Sci USA 86:6597–6601
White SH, Wimley WC, Selsted ME (1995) Structure, function, and membrane integration of defensins. Curr Opin Struct Biol 5:521–527
Wimley WC (2010) Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5:905–917
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Yount NY, Yeaman MR (2004) Multidimensional signatures in antimicrobial peptides. Proc Natl Acad Sci USA 101:7363–7368
Acknowledgements
This work was supported by NIH grants GM060000, GM068619 and GM095930. We dedicate this review to our advisor Stephen H. White, in whose laboratory we completed our first AMP research projects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wimley, W.C., Hristova, K. Antimicrobial Peptides: Successes, Challenges and Unanswered Questions. J Membrane Biol 239, 27–34 (2011). https://doi.org/10.1007/s00232-011-9343-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-011-9343-0