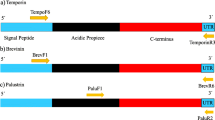
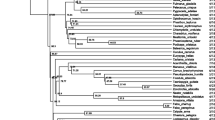
Abstract
Antimicrobial peptides are highly diverse pathogen-killing molecules. In many taxa, their evolution is characterized by positive selection and frequent gene duplication. It has been proposed that genes encoding antimicrobial peptides might be subject to balancing selection and/or an enhanced mutation rate, but these hypotheses have not been well evaluated because allelic variation has rarely been studied at antimicrobial peptide loci. We present an evolutionary analysis of novel antimicrobial peptide genes from leopard frogs, Rana. Our results demonstrate that a single genome contains multiple homologous copies, among which there is an excess of nonsynonymous nucleotide site divergence relative to that expected from synonymous site divergence. Thus, we confirm the trends of recurrent duplication and positive selection. Allelic variation is quite low relative to interspecies divergence, indicating a recent positive selective sweep with no evidence of balancing selection. Repeated gene duplication, rather than a balanced maintenance of divergent allelic variants at individual loci, appears to be how frogs have responded to selection for a diverse suite of antimicrobial peptides. Our data also support a pattern of enhanced synonymous site substitution in the mature peptide region of the gene, but we cannot conclude that this is due to an elevated mutation rate.



Similar content being viewed by others
References
Ali MF, Lips KR, Knoop FC, Fritzsch B, Miller C, Conlon JM (2002) Antimicrobial peptides and protease inhibitors in the skin secretions of the crawfish frog, Rana areolata. Biochim Biophys Acta 1601:55–63
Altizer S, Harvell D, Friedle E (2003) Rapid evolutionary dynamics and disease threats to biodiversity. Trends in Ecology and Evolution 18:589–596
Bakker EG, Toomajian C, Kreitman M, Bergelson J (2006) A genome-wide survey of R gene polymorphisms in Arabidopsis. Plant Cell 18:1803–1818
Basir YJ, Knoop FC, Dulka J, Conlon JM (2000) Multiple antimicrobial peptides and peptides related to bradykinin and neuromedin N isolated from skin secretions of the pickerel frog, Rana palustris. Biochim Biophys Acta 1543:95–105
Bergelson J, Kreitman M, Stahl EA, Tian D (2001) Evolutionary dynamics of plant R-genes. Science 292:2281–2285
Bulmer MS, Crozier RH (2004) Duplication and diversifying selection among termite antifungal peptides. Mol Biol Evol 21:2256–2264
Chen T, Farragher S, Bjourson AJ, Orr DF, Rao P, Shaw C (2003) Granular gland transcriptomes in stimulated amphibian skin secretions. Biochem J 371:125–130
Chen T, Zhou M, Rao P, Walker B, Shaw C (2006) The Chinese bamboo leaf odorous frog (Rana (Odorrana) versabilis) and North American Rana frogs share the same families of skin antimicrobial peptides. Peptides 27:1738–1744
Chinchar VG, Bryan L, Silphadaung U, Noga E, Wade D, Rollins-Smith L (2004) Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology 323:268–275
Clark AG, Wang L (1997) Molecular population genetics of Drosophila immune system genes. Genetics 147:713–724
Conlon JM, Halverson T, Dulka J, Platz JE, Knoop FC (1999) Peptides with antimicrobial activity of the brevinin-1 family isolated from skin secretions of the southern leopard frog, Rana sphenocephala. J Peptide Res 54:522–527
Conlon JM, Sonnevend Á, Patel M, Davidson C, Nielsen PF, Pál T, Rollins-Smith LA (2003) Isolation of peptides of the brevinin-1 family with potent candidacidal activity from the skin secretions of the frog Rana boylii. J Peptide Res 62:207–213
Conlon JM, Kolodziejek J, Nowotny N (2004) Antimicrobial peptides from ranid frogs: taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim Biophys Acta 1696:1–14
Cronn R, Cedroni M, Haselkorn T, Grover C, Wendel JF (2002) PCR-mediated recombination in amplification products derived from polyploidy cotton. Theor Appl Genet 104:482–489
Crump D, Lean D, Trudeau VL (2002) Octylphenol and UV-B radiation alter larval development and hypothalamic gene expression in the leopard frog (Rana pipiens). Environ Health Perspect 110:277–284
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife – threats to biodiversity and human health. Science 287:443–449
Duda TF Jr, Vanhoye D, Nicolas P (2002) Roles of diversifying selection and coordinated evolution in the evolution of amphibian antimicrobial peptides. Mol Biol Evol 19:858–864
Garrigan D, Hedrick PW (2003) Perspective: detecting adaptive molecular polymorphism: lessons from the MHC. Evolution 57:1707–1722
Goraya J, Wang Y, Li Z, O’Flaherty M, Knoop FC, Platz JE, Conlon JM (2000) Peptides with antimicrobial activity from four different families isolated from the skins of the North American frogs Rana luteiventris, Rana berlandieri, and Rana pipiens. Eur J Biochem 267:894–900
Hillis DM, Wilcox TP (2005) Phylogeny of the New World true frogs (Rana). Mol Phylogenet and Evol 34:299–314
Hoffman EA, Blouin MS (2004) Evolutionary history of the northern leopard frog: reconstruction of phylogeny, phylogeography, and historical changes in population demography from mitochondrial DNA. Evolution 58:145–159
Horikawa R, Parka DS, Herring PL, Pisano JJ (1985) Pipinins: new mast cell degranulating peptides from Rana pipiens. Fed Proc 44:695
Hudson RR, Kreitman M, Aguadé M (1987) A test of neutral molecular evolution based on nucleotide data. Genetics 116:153–159
Ironside JE, Filatov DA (2005) Extreme population structure and high interspecific divergence of the silene Y chromosome. Genetics 171:705–713
Kwon SY, Carlson BA, Park JM, Lee BJ (2000) Structural organization and expression of the gaegurin 4 gene of Rana rugosa. Biochim Biophys Acta 1492:185–190
Lutz GJ, Cuizon DB, Ryan AF, Lieber RL (1998) Four novel myosin heavy chain transcripts define a molecular basis for muscle fibre types in Rana pipiens. J Physiol 508:667–80
Lynn DJ, Higgs R, Gaines S, Tierney J, James T, Lloyd AT, Fares MA, Mulcahy G, O’Farrelly C (2004) Bioinformatic discovery and initial characterisation of nine novel antimicrobial peptide genes in the chicken. Immunogenetics 56:170–177
Marenah L, Flatt PR, Orr DF, Shaw C, Abdel-Wahab YHA (2005) Characterization of naturally occurring peptides in the skin secretion of Rana pipiens frog reveal pipinin-1 as the novel insulin-releasing agent. J Pept Res 66:204–210
Maxwell AI, Morrison GM, Dorin JR (2003) Rapid sequence divergence in mammalian β-defensins by adaptive evolution. Mol Immunol 40:413–421
Maynard Smith JM (1992) Analyzing the mosaic structure of genes. J Mol Evol 34:126–129
Morjan CL, Rieseberg LH (2004) How species evolve collectively: implications of gene flow and selection for the spread of advantageous alleles. Mol Ecol 13:1341–1356
Park JM, Jung J-E, Lee BJ (1994) Antimicrobial peptides from the skin of a Korean frog, Rana rugosa. Biochem Biophys Res Commun 205:948–954
Perron GG, Zasloff M, Bell G (2006) Experimental evolution of resistance to an antimicrobial peptide. Proc Biol Sci 273:251–256
Peschel A, Sahl H-G (2006) The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4:529–536
Piertney SB, Oliver MK (2006) The evolutionary ecology of the major histocompatibility complex. Heredity 96:7–21
Rollins-Smith LA, Conlon JM (2005) Antimicrobial peptide defenses against chytridiomycosis, an emerging infectious disease of amphibian populations. Dev Comp Immunol 29:589–598
Rollins-Smith LA, Carey C, Longcore J, Doersam JK, Boutte A, Bruzgal JE, Conlon JM (2002) Activity of antimicrobial skin peptides from ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Dev Comp Immunol 26:471–479
Rozas J, Sánchez-DelBarrio JC, Messeguer X, Rozas R (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19:2496–2497
Schroder JM (1999) Epithelial peptide antibiotics. Biochem Pharmacol 57:121–134
Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, Welsh MJ, Casavant TL, McCray PB Jr (2002) Discovery of five conserved β-defensin gene clusters using a computational search strategy. Proc Natl Acad Sci USA 99:2129–2133
Semple CAM, Rolfe M, Dorin JR (2003) Duplication and selection in the evolution of primate β-defensin genes. Genome Biol 4:R31
Swofford DL (2002) PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods) Version 4.0b10. Sinauer Associates, Sunderland, MA
Tennessen JA (2005a) Molecular evolution of animal antimicrobial peptides: widespread moderate positive selection. J Evol Biol 18:1387–1394
Tennessen JA (2005b) Enhanced synonymous site divergence in positively selected vertebrate antimicrobial peptide genes. J Mol Evol 61:445–455
Tiffin P, Hacker R, Gaut BS (2004) Population genetic evidence for rapid changes in intraspecific diversity and allelic cycling of a specialist defense gene in Zea. Genetics 168:425–434
Wakeley J (1999) Nonequilibrium migration in human history. Genetics 153: 1863–1871
Won H-S, Kim SS, Jung S-J, Son W-S, Lee B, Lee B-J (2004) Structure-activity relationships of antimicrobial peptides from the skin of Rana esculenta inhabiting Korea. Mol Cells 17:469–476
Vanhoye D, Bruston F, Nicolas P, Amiche M (2003) Antimicrobial peptides from hylid and ranin frogs originated from a 150-million year old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur J Biochem 270:2068–2081
Yang Z (1997) PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13:555–556
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Acknowledgments
We thank C. Criscione, E. Hoffman, and K. Field. This work was supported by a National Science Foundation Graduate Research Fellowship to J. Tennessen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tennessen, J.A., Blouin, M.S. Selection for Antimicrobial Peptide Diversity in Frogs Leads to Gene Duplication and Low Allelic Variation. J Mol Evol 65, 605–615 (2007). https://doi.org/10.1007/s00239-007-9045-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-007-9045-5