Abstract
Acute kidney injury (AKI) is a frequent complication after cardiopulmonary bypass surgery during infancy. Standard methods for evaluating renal function are not particularly sensitive nor are proximate indicators of renal dysfunction that allow intervention in real time. Near-infrared spectroscopy (NIRS) is a newer noninvasive technology that continuously evaluates regional oximetry and may correlate with renal injury and adverse outcomes after cardiac surgery in infants. This prospective observational study enrolled 40 infants (age, <12 months) undergoing biventricular repair. Continuous renal oximetry data were collected for the first 48 postoperative hours and correlated with postoperative course, standard laboratory data, and the occurrence of acute renal injury. Subjects with low renal oximetry (below 50% for >2 h) had significantly higher postoperative peak creatinine levels by 48 h (0.8 ± 0.4 vs. 0.52 ± 0.2; p = 0.003) and a higher incidence of AKI (50 vs. 3.1%; p = 0.003) than those with normal renal oximetry. These subjects also required more ventilator days and greater vasoactive support, and they had elevated lactate levels. Prolonged low renal near-infrared oximetry appears to correlate with renal dysfunction, decreased systemic oxygen delivery, and the overall postoperative course in infants with congenital heart disease undergoing biventricular repair.
Similar content being viewed by others
With improvements in surgical techniques and perioperative care, mortality associated with surgical repair or palliation of infants with congenital heart disease has steadily declined over the past few decades. As a result, recent emphasis has shifted toward minimizing complications and morbidities associated with cardiopulmonary bypass and infant heart surgery. In particular, acute kidney injury (AKI) is common after cardiopulmonary bypass in children, with a reported incidence of approximately 5–20% [7, 13, 14, 17, 20]. Furthermore, AKI is associated with prolonged hospitalization, poor outcome, and increased mortality [2, 6, 20, 27, 34].
Efforts to identify risk factors, earlier diagnosis, and prevention of AKI after cardiac surgery in infancy are of paramount importance. Most AKI classifications such as the Pediatric Renal-Injury-Failure-Loss-End (pRIFLE) stage [3, 4, 29] and the Acute Kidney Injury Network (AKIN) [22, 24] criteria rely on measures of renal dysfunction such as creatinine clearance and oliguria. Although changes in these parameters may reflect renal injury, they are relatively insensitive and late markers of AKI. Also, pRIFLE and AKIN criteria have not been validated after cardiac surgery in infants.
Recently, new urinary and serum biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL) [5, 8, 12, 25] and cystatin C [18, 31] have been proposed as earlier markers of kidney injury, potentially facilitating diagnosis and prompt treatment. Although these new biomarkers show some promise, they have not yet been validated in infants after heart surgery, and they do not allow for continuous monitoring of renal function.
Near-infrared spectroscopy (NIRS) is a new continuous, noninvasive technology that measures regional oximetry [30, 33]. The NIRS technology is based on the differential absorption of wavelengths of light by oxygenated and deoxygenated hemoglobin. The measured value represents the oxygen content within a local tissue bed.
Cerebral NIRS has been positively correlated with measurements of mixed venous saturation (SvO2) [1, 9, 26] and other surrogates of cardiac output.
Low cerebral oximetry (values <45–50% for more than 2 h) during or after cardiac surgery is associated with an increased risk of hypoxic–ischemic injury [32]. In pediatric patients undergoing cardiac surgery, low intraoperative cerebral oximetry has been correlated with decreased cognitive function at 1-year follow-up assessment [21]. In addition, in patients with hypoplastic left heart syndrome, low cerebral NIRS (<55%) in the initial 48 h postoperatively has been a significant predictor of an adverse outcome [28].
The clinical utility of somatic NIRS also has been investigated in a few studies. For example, abdominal NIRS correlated with gastric tonometry in infants after cardiac surgery and was shown to be a predictor for necrotizing enterocolitis in neonates [15]. In another study, multisite oximetry was correlated with lactate levels in patients undergoing surgery for congenital heart disease [10].
Changes in regional oximetry (including renal NIRS) also have been observed after delayed sternal closure and with pericardial tamponade [23]. However, to date, no studies to our knowledge correlate renal oximetry measurements with AKI in infants after cardiac surgery.
The primary objective of this study was to evaluate whether persistent low renal oximetry (<50% for more than 2 h) was associated with AKI (as defined by pRIFLE and other criteria) after cardiac surgery in infants.
Materials and Methods
Parents or legal guardians of all infants younger than 12 months undergoing planned biventricular repair with cardiopulmonary bypass were approached for consent. The exclusion criteria specified prematurity (postconceptional age, ≤35 weeks), known genetic or chromosomal disorder, functional single-ventricle anatomy, abnormal renal ultrasound, or preoperative renal insufficiency based on an abnormal serum creatinine for age.
Demographic information including age at operation, preoperative weight, gender, primary cardiac diagnosis, surgical procedure, Risk Adjustment for Congenital Heart Surgery (RACHS-1) [19] classification, length of cardiopulmonary bypass, aortic cross-clamp time, time to extubation, and circulatory arrest time was recorded.
In the operating room or immediately at arrival in the cardiothoracic intensive care unit postoperatively, a pediatric-sized oximetry sensor was placed on the right flank below the costovertebral angle overlying the right kidney. An INVOS 5100B oximeter (Somanetics Corp, Troy, MI, USA) was used to monitor renal oximetry. Regional oximetry was collected (in 20-s intervals) for the initial 48 h after surgery, and the primary caregivers were blinded to the results.
The patients were dichotomized into “low oximetry” or “normal oximetry” groups based on the total time (≥2 vs. <2 h, respectively) each patient spent below an oximetry threshold of 50%. This threshold was chosen based on previous cerebral oximetry data suggesting correlation of prolonged low oximetry with end-organ dysfunction [11, 32]. Comparisons of demographic, operative, postoperative, and hospital course data, including assessment of renal function and secondary clinical variables (e.g., lactate and inotropic score), were made between groups.
The primary outcome of AKI was defined as meeting “failure” under pRIFLE criteria (creatinine clearance, <35 ml/min) or having an absolute increase in creatinine by 0.4 mg/dl and at least 50%. This second definition of AKI was based on evolving data suggesting that small changes in creatinine may indicate kidney injury in infants undergoing cardiopulmonary bypass (CPB) [35] and that pRIFLE may not be appropriate in this population. Sentinel events, such as the need for extracorporeal membrane oxygenation (ECMO) or dialysis, and mortality also were recorded.
Physiologic data were expressed as mean ± standard deviation. An unpaired two-tailed t-test was used to compare continuous data between groups. Fisher’s exact test was used for dichotomous values.
Results
The study enrolled 40 neonates and infants undergoing cardiac surgery resulting in biventricular repair. The diagnoses and surgical repairs are listed in Table 1. The two most frequent diagnoses were tetralogy of Fallot (TOF) and d-transposition of the great arteries (d-TGA) (Table 1). No major intraoperative complications were found. The demographic characteristics are reported in Table 2. The mean age at operation was 81 days, and 65% of the patients were male.
The predefined criteria for low renal oximetry were met by eight patients as follows: TOF (n = 6), d-TGA + coarctation (n = 1), and truncus arteriosus (n = 1). The remaining 32 patients were classified in the normal renal oximetry group. On the average, the low oximetry group spent 13% (6 h) of the initial 48 postoperative hours below 50% compared with less than 1% (0.47 h) for the normal oximetry group (p < 0.005).
Comparisons of demographic and baseline clinical variables between groups are shown in Table 2. The groups did not differ statistically in age, weight, or RACHS-1 score. In addition, there was no statistical difference in CPB time, aortic cross-clamp time, or use of circulatory arrest between the cohorts.
One patient in the low oximetry group required ECMO support and eventually died on postoperative day 11 after failing to wean from ECMO support. Another patient in the normal oximetry group was resuscitated and fully recovered from a cardiac arrest.
The primary and secondary outcome data are shown in Table 3. A total of five patients (12.5%) experienced AKI based on pRIFLE creatinine criteria for “failure,” and 10 patients (25%) had an increase in creatinine by at least 0.4 mg/dl and 50%. Only one patient (low oximetry group) required renal replacement therapy. Findings showed that 63% of the patients (n = 5) with low renal oximetry experienced AKI based on our definition versus 16% (n = 5) of the patients with normal renal oximetry (p = 0.015).
According to pRIFLE, 50% of the patients (n = 4) with low oximetry compared with 3.1% of the patients (n = 1) with normal oximetry met the criteria for acute renal failure based on a creatinine clearance of less than 35 ml/min (p = 0.003). The low oximetry patients had a nearly twofold increase in length of mechanical ventilation compared with normal oximetry patients (7.6 vs. 4.3, respectively; p = 0.009), but total hospitalization days did not differ between the two groups (Table 3).
Additionally, peak creatinine was significantly higher in the low oximetry group than in the normal group (0.83 ± 0.4 vs. 0.52 ± 0.2; p = 0.003). The low oximetry cohort also had higher average lactate levels (3.0 ± 2.5 vs. 1.5 ± 0.7; p = 0.004) and the maximum vasoactive inotropic score (VIS) [16] (23.6 ± 17 vs. 13.8 ± 8.8; p = 0.03) within the first 24 h compared with the normal oximetry group (Table 3), suggesting a more critical clinical status.
Discussion
Acute kidney injury continues to be a significant complication of CPB and cardiac surgery in children and infants, resulting in increased morbidity and mortality [6, 7, 27]. Consequently, early detection and correction of aberrations in kidney function and perfusion are essential for the management of these critically ill patients. Not only are the criteria for the diagnosis of AKI in this population evolving, but new diagnostic tools and techniques also are continually under investigation. To our knowledge, this is the first study to associate low renal oximetry with acute kidney injury in pediatric patients undergoing biventricular repair for congenital heart disease.
Typically, renal function is evaluated with serum creatinine level (or creatinine clearance) and urine output, leading to the development of diagnostic criteria such as pRIFLE and AKIN (acute kidney injury network). Unfortunately, changes in both of these markers may be delayed as much as 48–72 h after renal injury.
The low renal oximetry values in this study were observed mainly in the first 24 h postoperatively, with serum creatinine typically peaking by 48 h. Thus, renal NIRS may have the unique ability of providing early real-time data regarding regional oxygenation that may serve an important role in monitoring renal perfusion and the effectiveness of interventions aimed at preventing or reversing renal injury.
Notably, cerebral oximetry measured in the study patients did not correlate as strongly with AKI or low renal oximetry, suggesting the importance of regional monitoring.
Although many studies analyzing regional oximetry are focused on the acute or chronic effects of ischemia on end-organ function, there may be an added benefit of monitoring regional oximetry above and beyond the traditional clinical parameters typically monitored in an intensive care unit (ICU) setting.
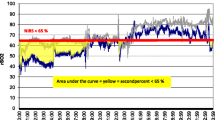
In our study, one patient (in the normal oximetry group) had an acute cardiorespiratory arrest during the monitoring period after an initial 24 h of stability. This patient displayed no apparent signs of compromise based on standard clinical parameters or intermittent laboratory tests (Fig. 1). However, 5 h before the arrest, a significant drop in regional oximetry was observed, which persisted until the arrest. It was not until after the arrest episode that alterations in his vital signs and serum markers of impaired oxygenation (i.e., lactate, HCO3) were clearly identified. This change in oximetry was reversed with resuscitation, as were the standard clinical parameters (Fig. 1). Although this is only one case example, it suggests that continuous somatic oximetry may prove useful for identifying silent changes in oxygen delivery earlier than standard monitoring approaches and may be used to prevent cardiovascular collapse.
Case of an 8-month-old boy with tetralogy of Fallot after complete repair. The boy was extubated, with normal blood pressure, normal serum bicarbonate and lactate levels, and renal oximetry values above 80%. As shown in the graph, renal oximetry dropped 13 h after surgery without significant changes to the other parameters, and 5½ h later, the boy had a cardiopulmonary arrest (arrow). Elevation in lactate and decreases in bicarbonate and blood pressure then became apparent. After resuscitation, all parameters normalized
This study was limited by its small sample size and by the enrollment criteria that included only complete biventricular repairs. A larger cohort including more complex congenital heart lesions (e.g., functional single ventricles) may have had a higher incidence of AKI, allowing for more robust statistical analysis.
Unfortunately, although estimated thresholds for “normal” regional oximetry exist in fully saturated patients, we are not aware of any criteria for abnormal regional oximetry in hypoxemic patients with palliated congenital heart disease and residual right-to-left shunts. Therefore, we chose not to include these patients in this initial study.
In addition, no consensus definition exists for AKI in infants and children undergoing cardiac surgery. Application of pRIFLE or AKIN criteria to this patient population may not be appropriate because the mechanism for acute kidney injury (i.e., CPB) is very different from that in other patient populations for which these criteria may be useful. Investigations are currently underway at our institution to define AKI more carefully in infants and children undergoing CPB surgery.
In summary, low renal oximetry, at least as defined in this study, appears to be associated with AKI, worse clinical status, and increased length of mechanical ventilation in neonates and infants after biventricular repair of congenital heart disease. Persistently low renal oximetry also may be a harbinger of impending cardiovascular collapse in these same subjects. As a noninvasive technology that provides continuous real-time output, regional oximetry may eventually prove to be an essential part of postoperative care in the pediatric cardiac intensive care unit.
Additional prospective randomized controlled trials are being designed to evaluate whether interventions aimed at improving regional oximetry can prevent the development of end-organ dysfunction. These types of prospective interventional studies are absolutely crucial toward understanding the true clinical utility of this innovative monitoring approach.
References
Abdul-Khaliq H, Troitzsch D, Berger F, Lange PE (2000) Regional transcranial oximetry with near-infrared spectroscopy (NIRS) in comparison with measuring oxygen saturation in the jugular bulb in infants and children for monitoring cerebral oxygenation. Biomed Tech (Berl) 45:328–332
Abou El-Ella RS, Najm HK, Godman M, Kabbani MS (2008) Acute renal failure and outcome of children with solitary kidney undergoing cardiac surgery. Pediatr Cardiol 29:614–618
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035
Askenazi DJ, Bunchman TE (2007) Pediatric acute kidney injury: the use of the RIFLE criteria. Kidney Int 71:963–964
Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S (2007) Neutrophil gelatinase-associated lipocalin (NGAL) correlations with cystatin C, serum creatinine, and eGFR in patients with normal serum creatinine undergoing coronary angiography. Nephrol Dial Transplant 22:295–296
Baskin E, Saygili A, Harmanci K, Agras PI, Ozdemir FN, Mercan S, Tokel K, Saatci U (2005) Acute renal failure and mortality after open heart surgery in infants. Ren Fail 27:557–560
Baxter P, Rigby ML, Jones OD, Lincoln C, Shinebourne EA (1985) Acute renal failure following cardiopulmonary bypass in children: results of treatment. Int J Cardiol 7:235–243
Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P (2008) Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 3:665–673
Bhutta AT, Ford JW, Parker JG, Prodhan P, Fontenot EE, Seib PM, Stroope BI, Frazier EA, Schmitz ML, Drummond-Webb JJ, Morrow WR (2007) Noninvasive cerebral oximeter as a surrogate for mixed venous saturation in children. Pediatr Cardiol 28:34–41
Chakravarti SB, Mittnacht AJ, Katz JC, Nguyen K, Joashi U, Srivastava S (2009) Multisite near-infrared spectroscopy predicts elevated blood lactate level in children after cardiac surgery. J Cardiothorac Vasc Anesth 23:663–667
Dent CL, Spaeth JP, Jones BV, Schwartz SM, Glauser TA, Hallinan B, Pearl JM, Khoury PR, Kurth CD (2005) Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg 130:1523–1530
Devarajan P (2008) Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl 241:89–94
Dittrich S, Kurschat K, Dahnert I, Vogel M, Muller C, Alexi-Meskishvili V, Lange PE (2000) Renal function after cardiopulmonary bypass surgery in cyanotic congenital heart disease. Int J Cardiol 73:173–179
Dittrich S, Priesemann M, Fischer T, Boettcher W, Muller C, Alexi-Meskishvili V, Lange PE (2002) Circulatory arrest and renal function in open heart surgery on infants. Pediatr Cardiol 23:15–19
Fortune PM, Wagstaff M, Petros AJ (2001) Cerebrosplanchnic oxygenation ratio (CSOR) using near-infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med 27:1401–1407
Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, Charpie JR, Hirsch JC (2010) Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 11:234–238
Gomez-Campdera FJ, Maroto-Alvaro E, Galinanes M, Garcia E, Duarte J, Rengel-Aranda M (1988) Acute renal failure associated with cardiac surgery. Child Nephrol Urol 9:138–143
Heise D, Waeschle RM, Schlobohm J, Wessels J, Quintel M (2009) Utility of cystatin C for assessment of renal function after cardiac surgery. Nephron Clin Pract 112:c107–c114
Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI (2002) Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 123:110–118
Kist-van Holthe tot Echten JE, Goedvolk CA, Doornaar MB, van der Vorst MM, Bosman-Vermeeren JM, Brand R, van der Heijden AJ, Schoof PH, Hazekamp MG (2001) Acute renal insufficiency and renal replacement therapy after pediatric cardiopulmonary bypass surgery. Pediatr Cardiol 22:321–326
Kussman BD, Wypij D, Laussen PC, Soul JS, Bellinger DC, DiNardo JA, Robertson R, Pigula FA, Jonas RA, Newburger JW (2010) Relationship of intraoperative cerebral oxygen saturation to neurodevelopmental outcome and brain magnetic resonance imaging at 1 year of age in infants undergoing biventricular repair. Circulation 122:245–254
Lopes JA, Jorge S, Silva S, de Almeida E, Abreu F, Martins C, Alves do Carmo J, Lacerda JF, Prata MM (2007) Prognostic utility of the acute kidney injury network (AKIN) criteria for acute kidney injury in myeloablative haematopoietic cell transplantation. Bone Marrow Transplant 40:1005–1006
Maher KO, Phelps HM, Kirshbom PM (2009) Near infrared spectroscopy changes with pericardial tamponade. Pediatr Crit Care Med 10:e13–e15
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31
Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P (2005) Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365:1231–1238
Nagdyman N, Fleck T, Schubert S, Ewert P, Peters B, Lange PE, Abdul-Khaliq H (2005) Comparison between cerebral tissue oxygenation index measured by near-infrared spectroscopy and venous jugular bulb saturation in children. Intensive Care Med 31:846–850
Pedersen KR, Hjortdal VE, Christensen S, Pedersen J, Hjortholm K, Larsen SH, Povlsen JV (2008) Clinical outcome in children with acute renal failure treated with peritoneal dialysis after surgery for congenital heart disease. Kidney Int Suppl 108:S81–S86
Phelps HM, Mahle WT, Kim D, Simsic JM, Kirshbom PM, Kanter KR, Maher KO (2009) Postoperative cerebral oxygenation in hypoplastic left heart syndrome after the Norwood procedure. Ann Thorac Surg 87:1490–1494
Plotz FB, Bouma AB, van Wijk JA, Kneyber MC, Bokenkamp A (2008) Pediatric acute kidney injury in the ICU: an independent evaluation of pRIFLE criteria. Intensive Care Med 34:1713–1717
Pollard V, Prough DS, DeMelo AE, Deyo DJ, Uchida T, Stoddart HF (1996) Validation in volunteers of a near-infrared spectroscope for monitoring brain oxygenation in vivo. Anesth Analg 82:269–277
Randers E, Kristensen JH, Erlandsen EJ, Danielsen H (1998) Serum cystatin C as a marker of the renal function. Scand J Clin Lab Invest 58:585–592
Slater JP, Guarino T, Stack J, Vinod K, Bustami RT, Brown JM III, Rodriguez AL, Magovern CJ, Zaubler T, Freundlich K, Parr GV (2009) Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg 87:36–44 (discussion 44–35)
Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG (2001) Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J Appl Physiol 90:511–519
Welke KF, Dearani JA, Ghanayem NS, Beland MJ, Shen I, Ebels T (2008) Renal complications associated with the treatment of patients with congenital cardiac disease: consensus definitions from the Multi-Societal Database Committee for Pediatric and Congenital Heart Disease. Cardiol Young 18(Suppl 2):222–225
Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, Hyder A, Alkandari O (2009) A small postoperative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int 76:885–892
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Owens, G.E., King, K., Gurney, J.G. et al. Low Renal Oximetry Correlates With Acute Kidney Injury After Infant Cardiac Surgery. Pediatr Cardiol 32, 183–188 (2011). https://doi.org/10.1007/s00246-010-9839-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-010-9839-x