Abstract
The utility of the rhesus macaque as an animal model in both HIV vaccine development and pathogenesis studies necessitates the development of accurate and efficient major histocompatibility complex (MHC) genotyping technologies. In this paper, we describe the development and application of allele-specific polymerase chain reaction (PCR) amplification for the simultaneous detection of eight MHC class I alleles from the rhesus macaque (Macaca mulatta) of Indian descent. These alleles were selected, as they have been implicated in the restriction of CD8+ T cell epitopes of simian immunodeficiency virus (SIV). Molecular typing of Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17 was conducted in a high throughput fashion using genomic DNA. Our amplification strategy included a conserved internal control target to minimize false negative results and can be completed in less than 5 h. We have genotyped over 4,000 animals to establish allele frequencies from colonies all over the western hemisphere. The ability to identify MHC-defined rhesus macaques will greatly enhance investigation of the immune responses, which are responsible for the control of viral replication. Furthermore, application of this technically simple and accurate typing method should facilitate selection, utilization, and breeding of rhesus macaques for AIDS virus pathogenesis and vaccine studies.
Similar content being viewed by others
Introduction
With the number of individuals globally infected with human immunodeficiency virus (HIV) at greater than 40 million and rising (UNAIDS/WHO AIDS Epidemic Update, December 2006), few biomedical priorities are more urgent than the effort to produce an effective AIDS vaccine. Induction of vaccine-induced broadly reactive neutralizing antibody response has proven very difficult due primarily to the diversity of the viral envelope protein (Burton et al. 2004; Gilbert et al. 2005). Consequently, current vaccine initiatives are focused on engendering potent cellular immune responses capable of controlling viral replication (Koff et al. 2006).
Certain major histocompatibility complex (MHC) class I alleles have been associated with control of HIV replication (Kaslow et al. 1996; McNeil et al. 1996; Carrington et al. 1999; Migueles et al. 2000; Carrington and O’Brien 2003; Kiepiela et al. 2004). However, the diversity of HIV and the dramatic polymorphism in the human MHC class I loci make it very difficult to define those immune responses which are beneficial. Simian immunodeficiency virus (SIV) infection of macaques is the best available animal model for defining those MHC-restricted immune responses critical to control of HIV pathogenesis (Nathanson et al. 1999; Hirsch and Lifson 2000; Sibal and Samson 2001).
As the genomic organization of the MHC is similar in rhesus macaques (Macaca mulatta) and humans (Kelley et al. 2005; Bontrop 2006), the rhesus macaque has been used extensively in biomedical research. While rhesus macaques express orthologues of MHC class I loci homologous to the HLA-A and HLA-B loci (Miller et al. 1991; Boyson et al. 1996), phylogenetic analysis clearly indicates that the great majority of rhesus classical MHC class I alleles cluster outside of and do not appear to be related to any of the classical human class I lineages (Boyson et al. 1996). Moreover, in contrast to the limited three highly polymorphic HLA-A, HLA-B, and HLA-C loci per haplotype in humans, any given rhesus chromosome can contain from 4 up to 14 functional class I loci (Daza-Vamenta et al. 2004; Otting et al. 2005; Shiina et al. 2006). Presently, there is no evidence for a locus similar to HLA-C in the rhesus macaque, suggesting that the HLA-C locus is of a fairly recent origin in humans (Watkins et al. 1988; Boyson et al. 1996). While much is yet to be elucidated about the MHC of the rhesus macaque relative to the human, it appears that each species has evolved different, yet effective, strategies to ensure that the critical function of the MHC is maintained in the face of numerous pathogens (Otting et al. 2005). Humans display extensive class I allelic polymorphism, whereas the rhesus macaque relies on many configurations with regard to the number and combination of loci.
To support the most efficient utilization of animal resources and to maximize the understanding of immune responses in SIV vaccine and viral pathogenesis studies, MHC typing technologies must be developed. To that end, we have directed our initial development efforts in MHC typing to those class I alleles that have been shown to functionally present viral epitopes. The Supplemental Table lists the eight MHC class I molecules of the rhesus macaque, Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17, and summarizes information regarding the CD8+ T cell epitopes derived from various SIV and SIV/HIV (SHIV) strains reported to be presented by these molecules. These restricted epitopes are derived from amino acid sequences encompassing the Gag, Pol, Env, Tat, Nef, Vif, Vpx, and Vpr proteins of these SIV and SHIV strains. In this present study, we describe a molecular genotyping method that simultaneously and specifically identifies Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17 from rhesus macaques of Indian descent. This allele-specific polymerase chain reaction (PCR) amplification is accurate, efficient, cost effective, and relatively straightforward, facilitating its adoption in other laboratories. This typing methodology has permitted the determination of frequencies for these eight MHC class I alleles from a significant number of rhesus animals from multiple cohorts around the western hemisphere. This technique will facilitate a greater understanding of the immune responses engendered by SIV infection during vaccine and viral pathogenesis studies, along with the better selection, utilization, and breeding of Indian rhesus macaques.
Materials and methods
Animals and samples
Animals were maintained at the Wisconsin National Primate Research Center (University of Wisconsin–Madison, Madison, WI), an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALACI) accredited facility. The animals were cared for according to the US animal welfare regulations and guidelines of the University of Wisconsin–Madison Institutional Animal Care and Use Committee.
Animals from all other institutions for which cell or blood samples were sent with requests for MHC class I typing were maintained and cared for according to the regulations and guidelines established at their respective facilities.
DNA extraction
Genomic DNA was isolated from a maximum of 3.0 × 106 peripheral blood mononuclear cells or 500 μl ethylenediaminetetraacetic acid (EDTA) anti-coagulated whole blood or buffy coat using the MagNA Pure LC system (Roche Applied Science, Indianapolis, IN) and the MagNA Pure LC DNA Isolation–Large Volume protocol (version 3.0) according to manufacturers guidelines. The elution volume of extracted DNA was 200 μl of MagNA Pure LC DNA Isolation–Large Volume elution buffer. DNA concentrations (ng/μl) and Abs 260 nm/Abs 280 nm ratios were determined using a NanoDrop UV Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Extracted genomic DNA with Abs 260 nm/Abs280 nm ratios ranging from 1.7–2.1 were diluted to15 ng/μl in a 40-μl volume using DNAse-, RNAse-free water (Invitrogen, Carlsbad, CA) for genotyping.
MHC genotyping by allele-specific amplification
All PCR primers were synthesized and provided salt-free by Operon Biotechnologies (Huntsville, AL). Allele-specific primer sequences and their location within the complementary DNA (cDNA) sequences of Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17 are noted in Table 1; primer locations within the cDNA sequences were determined by aligning Mamu-A*02, Mamu-A*08, Mamu-A*11 to Mamu-A*01 and Mamu-B*03, Mamu-B*04, Mamu-B*17 to Mamu-B*01. Primers targeting highly conserved sequences of Mamu-DRB exon 2 (forward: 5′ GCC TCG AGT GTC CCC CCA GCA CGT TTC 3′; reverse: 5′ GCA AGC TTT CAC CTC GCC GCT G 3′), yielding a ∼300-base-pair amplification product, were included as an internal control in all typing reactions (Knapp et al. 1997a, b). Working stocks of primer mixes specific for the individual rhesus class I alleles and the Mamu-DRB internal control, at the concentrations listed in Table 1, were pre-aliquoted in a volume of 12 μl into Low Profile 96 well polycarbonate trays (Continental Lab Products, San Diego, California). Working primer mixes for Mamu-A*02 and Mamu-A*11 with the Mamu-DRB internal control primers also contained 500 mM of molecular grade betaine (Sigma-Aldrich, St. Louis, MO). Typing trays containing working primer mixes were sealed with SilverSeal I foil plate seals (Continental Lab Products) and stored at 4°C for up to 2 weeks.
A 5× PCR reaction buffer at pH 9.5 consisting of 300 mM TRIS hydrochloride, 10 mM magnesium chloride, and 75 mM ammonium sulfate was prepared with molecular grade reagents (Fisher Scientific, Pittsburgh, PA) and DNAse-, RNAse-free Water (Invitrogen). Once in solution, 5× PCR reaction buffer was filter sterilized, divided into 8 ml aliquots in sterile 15 ml Corning conical tubes (Fisher Scientific) and stored at −20°C for up to 12 months. The 5× PCR reaction buffer was thawed at 50–60°C with vigorous vortexing. A PCR master mix was made by combining an 8.0-ml aliquot of thawed 5× PCR reaction buffer with 1,640 μl of DNAse-, RNAse-free Water (Invitrogen), 2.0 ml of glycerol (Sigma-Aldrich), 320 μl of 25-mM deoxynucleotide triphosphates (Promega, Madison, WI), and 40 μl of 10 mg/ml cresol red (Sigma-Aldrich). For each set of eight rhesus MHC class I allele typing reactions to be conducted for a given sample, 40 μl of extracted genomic DNA diluted to 15 ng/μl was added with gentle mixing to 64 μl PCR master mix to which 10 U of Platinum Taq DNA polymerase (5 U/μl; Invitrogen) had been added. Twelve microliters of PCR master mix with Platinum Taq DNA polymerase, and diluted genomic DNA was added to each primer mix pre-aliquoted into a Low Profile 96 well polycarbonate trays (Continental Lab Products). Trays were covered and sealed with SilverSeal I foil plate seals (Continental Lab Products) before thermal cycling.
Thermal cycling was conducted on a Tetrad™ thermal cycler (BioRad, Hercules, CA; formerly MJ Research, Cambridge, MA) under the following conditions: an initial 1-min denaturation at 96.0°C, followed by six cycles of 96.0°C denaturation for 25 s, 67.9°C annealing for 50 s and 45 s of elongation at 72.0°C; six cycles of 96.0°C denaturation for 25 s, 66.4°C annealing for 50 s and a 45-s elongation at 72.0°C; five cycles of 96.0°C denaturation for 25 s, 66.0°C annealing for 60 s (during which the annealing temperature was decreased 1.0°C for each of the five cycles) and a 45-s extension at 72.0°C; and finally 16 cycles of 96.0°C denaturation for 25 s, 63.0°C annealing for 50 s and 45 s of elongation at 72.0°C, followed by a final extension at 72.0°C for 10 min and a terminal hold at 25.0°C.
Subsequently, products resulting from PCR amplification were electrophoresed on 2.0% agarose (low EEO, molecular grade, Fisher Scientific) gels containing ethidium bromide (molecular grade, Fisher Scientific) on Owl Centipede separation systems (Fisher Scientific) at a constant voltage (230 V) for 17 min in 0.5× sodium borate buffer (Brody and Kern 2004). Gels were visualized and electronically documented using a fluorescent imaging system (Alpha Innotech, San Leandro, CA). Amplification products detected after electrophoresis were analyzed relative to a 100-bp DNA ladder (Invitrogen) for the presence of Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17 allele-specific amplicons (sizes are listed in Table 1). In addition to allele-specific amplicons, genotyping reactions were considered valid by the presence of the required internal control product. Additionally, known positive control DNA was included for each allele-specificity with each batch of genotyping reactions.
Results
PCR amplification utilizing sequence-specific priming (PCR-SSP) is currently the method of choice for intermediate (specificity for multiple alleles sharing primer targeted polymorphisms) or high (allele level) resolution HLA typing in many clinical histocompatibility laboratories (Olerup and Zetterquist 1991, 1992). This allele-specific PCR amplification methodology involves designing at least one or preferably both primers so that they will permit amplification based on the 3′-mismatch principle (Welsh and Bunce 1999). A single mismatch at the 3′ terminus of a primer will prevent enzymatic extension by DNA polymerase. We have aligned MHC class I cDNA sequences derived from the rhesus macaque of Indian descent submitted to GenBank (alignments not shown; see restricting allele accession numbers referenced in Table 1). Using these MHC class I allele alignments, primers targeting polymorphisms that are unique to and would provide specific amplification of the Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17 alleles were designed and are listed in Table 1. The DNA sequences targeted by the forward and reverse primers are located in exons 2, 3, or 4 because these exons contain highly variable regions capable of discriminating among class I alleles of the rhesus macaque. Whenever possible, the 3′ terminal region of each primer targets a specific nucleotide polymorphism unique to the MHC class I allele to maximize the specificity of the reaction. Furthermore, DNA sequences targeted by allele-specific primers were deliberately selected to amplify a broad range of intervening sequence. This strategy facilitates verification of allele-specificity by DNA sequencing of amplification products and allows for the detection of new alleles. In addition, the allele-specific product size minimizes any potential conflict in interpretation of these amplification products relative to non-specific products.
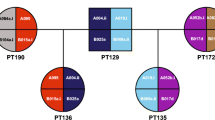
To maximize the throughput and efficiency of MHC genotyping, PCR-SSP typing reactions for the Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17 alleles were conducted under one set of universal thermal cycling parameters. These were optimized collectively under parallel reaction conditions such as buffer pH, magnesium ion concentration, concentrations of allele-specific and internal control primers, amount of input genomic DNA, and amount of Taq DNA polymerase (data not shown). PCR-SSP genotyping of these eight alleles has been authenticated by the production and sequencing of cDNA libraries and the direct sequencing of various allele-specific amplicons from a representative variety of both related and unrelated animals. Although not all allele-specific genotyping reactions are equally robust, amplification of these eight alleles has been optimized to provide specific and definitive results under these universal conditions (Fig. 1a–d). Each of the pedigrees in this representative genotyping includes multiple family members from three successive generations. In Fig. 1a, the segregation of haplotypes containing Mamu-A*01, Mamu-A*08/Mamu-B*17, and Mamu-A*02/Mamu-B*17 is demonstrated. Similarly, the inheritances of Mamu-A*01 and Mamu-B*01, Mamu-A*08, and Mamu-B*03/Mamu-B*04, and Mamu-A*11/Mamu-B*17 are illustrated in Fig. 1b–d, respectively. The sizes of each of the allele-specific amplification products (Fig. 1a–d) correspond to that predicted based on the location of primers (Table 1). A set of primers yielding a ~300-bp product corresponding to conserved sequences of the rhesus DRB class II alleles was present in all PCR-SSP reactions as an internal control to detect the presence of an inhibitor of PCR amplification, minimizing the potential for false negative results.
a–d Representative MHC genotyping by allele-specific PCR amplification of multiple members of four unrelated families across three generations that collectively have the Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17 alleles. Positive genotyping results are shown under each animal ID. The internal control amplification product is present in each typing reaction, and each gel image contains a standard DNA molecular weight marker (std)
As summarized in Fig. 2, we have typed for Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17 using allele-specific PCR amplification and established allele frequencies for Indian rhesus macaques from a variety of cohorts around the western hemisphere. Interestingly, while similarities among these different populations were apparent, disparities in allele frequencies both within and among colonies were also evident. Mamu-A*01, Mamu-A*02, Mamu-A*08, and Mamu-B*01 were all detected at a high frequency, having an average frequency ranging from 20–30% across all the cohorts. However, there were some exceptions to these observations. The frequencies of Mamu-A*01 at the Ohio State facility and Mamu-B*01 at the Caribbean facility were approximately twofold lower than the average. At the California National Primate Research Center, the Mamu-A*08 frequency was threefold below the average of all colonies at only 9%. In general, Mamu-A*11 and Mamu-B*17 were present at only moderate levels with means of 4.0 and 11.3%, respectively. It is interesting to note that in the case of both Mamu-A*11 and Mamu-B*17, the Caribbean frequency falls to less than 2%. Moreover, the frequency of Mamu-B*17 was at least twofold higher at the Oregon National Primate Center relative to the average (11.3%) of all the groups. In all cases, Mamu-B*03 and Mamu-B*04 allele frequencies were consistently low, at 3% or less.
Frequencies of the Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17 alleles based on allele-specific amplification from genomic DNA of Indian rhesus macaques from various colonies. The institutions included are as follows; Wisconsin National Primate Research Center (WNPRC), Caribbean Primate Research Center (Carib PRC), Oregon National Primate Research Center (ONPRC), National Institutes of Health and National Cancer Institute (NIH/NCI), California National Primate Research Center (CNPRC), and The Ohio State University (Ohio State). Average frequency for each of the Indian rhesus class I alleles across all six institutions is shown above the bar graphs
Discussion
Elucidation of the role of cytotoxic T-lymphocytes in controlling HIV and SIV requires the definition of MHC class I molecules and the HIV and SIV peptides that they bind. The significance of MHC matching to transplantation has also promoted the development of HLA typing methodologies. Unfortunately, genotyping techniques for the MHC of the rhesus macaque have lagged far behind HLA typing in humans. In contrast to the almost 1,600 alleles described for the human class I HLA-A, HLA-B, and HLA-C loci as of December 2006 (Robinson et al. 2006), currently GenBank contains sequences for only about 130 macaque MHC class I alleles, primarily derived from captive-bred animals of Indian origin. Given the broad significance of the rhesus animal model and the fundamental role of the MHC in the immune response, it is not surprising that molecular-based typing assays using allele-specific amplification (Knapp et al. 1997a; Lobashevsky and Thomas 2000; Muhl et al. 2002) and reference strand conformational analysis (Tanaka-Takahashi et al. 2007) for various rhesus class I alleles have been previously described.
In this study, we described the development of unified thermal cycling conditions for the simultaneous PCR-SSP-based genotyping of eight MHC class I alleles, Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, and Mamu-B*17, from the rhesus macaque of Indian descent. All of these alleles have been implicated in the restriction of SIV CD8+ T cell epitopes. We previously reported primer sequences for these eight alleles (Horton et al. 2001; Schramm et al. 2002; Vogel et al. 2002; Loffredo et al. 2005). To improve the original PCR-SSP for Mamu-A*01 (Knapp et al. 1997b), primers were redesigned in a subsequent study (Schramm et al. 2002), followed by modification of the forward primer here to further increase amplification efficiency. However, it should be noted that these new primers have occasionally exhibited cross reactivity in amplifying the non-classical Mamu-AG*01. Mamu-A*01 is easily distinguished from Mamu-AG*01 by differences in product size. Elevated frequencies of Mamu-B*03 have caused us to redesign the reverse primer here to minimize false positive results. As demonstrated in our current genotyping design (Fig. 1a–d), this platform is specific, robust, and straightforward. Additionally, PCR-SSP enables high throughput analysis for all the eight or any combination of these alleles, requires inexpensive equipment, is cost effective, and is less technically demanding and labor intensive than other molecular methods. Our standard procedure using PCR-SSP amplification for the detection of these eight alleles requires little input genomic DNA (approximately 70 ng). In fact, depending on the allele-specificity, lower amounts of high-quality input genomic DNA (down to approximately 25 ng per reaction) yielded valid genotyping results (data not shown). Over the last 5 years, using these allele-specific PCR primers, we have performed over 60,000 reactions and have provided MHC class I typing to more than 60 different investigators at over 30 public and private institutions around the western hemisphere.
The cellular immune responses restricted by certain MHC class I molecules, specifically HLA-B*27 and HLA-B*57, have been associated with a protective benefit and control of HIV replication (Kaslow et al. 1996; McNeil et al. 1996; Migueles et al. 2000; Carrington and O’Brien 2003; Kiepiela et al. 2004). The MHC-defined Indian rhesus macaque infected with SIV provides an excellent model for understanding the influence of MHC class I alleles on the replication of this pathogenic virus. Infection of Indian rhesus macaques with SIVmac239, a molecularly cloned AIDS virus, has been the most widely evaluated animal model for HIV pathogenesis and vaccine studies (Bontrop and Watkins 2005). MHC typing of macaques is critical in identifying class I alleles, which restrict epitopes that engender cytotoxic T-cell lymphocytes (CTL) and in facilitating analyses of CTL responses after vaccination and/or during the course of SIV pathogenesis. With the exception of Mamu-B*17 at the Caribbean Primate Research Center, Mamu-A*01, Mamu-A*02, and Mamu-B*17 alleles were all present at moderate to high (10–27.5%) frequencies across various colonies (Fig. 2). The ability to type for these alleles has been beneficial to numerous studies. Mamu-A*01 was the first MHC class I allele described in rhesus macaques (Miller et al. 1991) and was subsequently found to be associated with moderate control of SIV replication (Zhang et al. 2002; Mothe et al. 2003). Mamu-A*01 SIV-derived epitopes have been thoroughly investigated (Allen et al. 1998, 2001). Among these epitopes, Gag181–189CM9 and Tat28–35SL8 are of particular interest. The immunodominant CTL response against Tat28–35SL8 selects for rapid escape mutants of SIV during the acute phase, while CTL against Gag181–189CM9 select for mutants during the chronic phase (Allen et al. 2000). Presentation of epitopes by Mamu-A*02 has been thoroughly elucidated (Vogel et al. 2002; Loffredo et al. 2004), with Nef159–167YY9 and Gag71–79GY9 having a similar relationship with Tat28–35SL8 and Gag181–189CM9 respectively, in terms of selection for rapidly or slowly escaping SIV mutants (Vogel et al. 2002). While Mamu-A*08 and Mamu-B*01 are also expressed at relatively high frequencies (Fig. 2), their role in SIV vaccination and pathogenesis studies remains unclear. Knowledge of epitope presentation by Mamu-A*08 has been limited thus far to a single epitope derived from envelope in SHIV, a chimeric SIV that contains the envelope of HIVHXBC2 (Voss and Letvin 1996). Although Mamu-B*01-restricted epitopes were initially reported (Yasutomi et al. 1995; Su et al. 2005), it has been difficult to make tetramers for these previously reported epitopes (CJ Miller, personal communication), and no immunogenic SIV-derived epitopes were identified for Mamu-B*01 in a subsequent analysis (Loffredo et al. 2005). Collectively, these observations suggest that Mamu-B*01 does not bind SIV-derived epitopes and has no effect on SIV disease progression. Mamu-A*11 and Mamu-B*17 both occur at average frequencies two- to fivefold lower than Mamu-A*01, Mamu-A*02, Mamu-A*08, and Mamu-B*01 (Fig. 2). Mamu-B*17 is associated with control of SIV replication (O’Connor et al. 2003; Yant et al. 2006), although inheritance of Mamu-B*17-containing haplotypes does not predict control of SIV (Wojcechowskyj et al. 2007). Mamu-A*11-presented epitopes have been defined systematically with three of them being cross-reactive with the mouse H-2 class I molecule Kk (Sette et al. 2005). Mamu-B*03 and Mamu-B*04 were linked to a small number of slow progressors (Evans et al. 1999a). The low frequency of Mamu-A*11, Mamu-B*03, and Mamu-B*04 may preclude them from being practical in future SIV studies.
While MHC-defined macaques are useful in vaccine development and pathogenesis studies, MHC genotyping may also provide some very valuable information in additional arenas. MHC typing will facilitate colony management, selective inbreeding of animals, in vitro fertilization programs, production of monozygotic twins, and selected full- or half-siblings. These developments will enhance and expand rhesus macaque resources for critical vaccine studies so that the correlates of immunity can be dissected.
References
Allen TM, Sidney J, del Guercio MF, Glickman RL, Lensmeyer GL, Wiebe DA, DeMars R, Pauza CD, Johnson RP, Sette A, Watkins DI (1998) Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J Immunol 160:6062–6071
Allen TM, O’Connor DH, Jing P, Dzuris JL, Mothe BR, Vogel TU, Dunphy E, Liebl ME, Emerson C, Wilson N, Kunstman KJ, Wang X, Allison DB, Hughes AL, Desrosiers RC, Altman JD, Wolinsky SM, Sette A, Watkins DI (2000) Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386–390
Allen TM, Mothe BR, Sidney J, Jing P, Dzuris JL, Liebl ME, Vogel TU, O’Connor DH, Wang X, Wussow MC, Thomson JA, Altman JD, Watkins DI, Sette A (2001) CD8(+) lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule mamu-A*01: implications for vaccine design and testing. J Virol 75:738–749
Bontrop RE (2006) Comparative genetics of MHC polymorphisms in different primate species: duplications and deletions. Hum Immunol 67:388–397
Bontrop RE, Watkins DI (2005) MHC polymorphism: AIDS susceptibility in non-human primates. Trends Immunol 26:227–233
Boyson JE, Shufflebotham C, Cadavid LF, Urvater JA, Knapp LA, Hughes AL, Watkins DI (1996) The MHC class I genes of the rhesus monkey. Different evolutionary histories of MHC class I and II genes in primates. J Immunol 156:4656–4665
Brody JR, Kern SE (2004) Sodium boric acid: a Tris-free, cooler conductive medium for DNA electrophoresis. Biotechniques 36:214–216
Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT (2004) HIV vaccine design and the neutralizing antibody problem. Nat Immunol 5:233–236
Carrington M, Nelson GW, Martin MP, Kissner T, Vlahov D, Goedert JJ, Kaslow R, Buchbinder S, Hoots K, O’Brien SJ (1999) HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283:1748–1752
Carrington M, O’Brien SJ (2003) The influence of HLA genotype on AIDS. Annu Rev Med 54:535–551
Daza-Vamenta R, Glusman G, Rowen L, Guthrie B, Geraghty DE (2004) Genetic divergence of the rhesus macaque major histocompatibility complex. Genome Res 14:1501–1515
Dzuris JL, Sidney J, Appella E, Chesnut RW, Watkins DI, Sette A (2000) Conserved MHC class I peptide binding motif between humans and rhesus macaques. J Immunol 164:283–291
Egan MA, Kuroda MJ, Voss G, Schmitz JE, Charini WA, Lord CI, Forman MA, Letvin NL (1999) Use of major histocompatibility complex class I/peptide/beta2M tetramers to quantitate CD8(+) cytotoxic T lymphocytes specific for dominant and nondominant viral epitopes in simian-human immunodeficiency virus-infected rhesus monkeys. J Virol 73:5466–5472
Evans DT, Knapp LA, Jing P, Mitchen JL, Dykhuizen M, Montefiori DC, Pauza CD, Watkins DI (1999a) Rapid and slow progressors differ by a single MHC class I haplotype in a family of MHC-defined rhesus macaques infected with SIV. Immunol Lett 66:53–59
Evans DT, O’Connor DH, Jing P, Dzuris JL, Sidney J, da Silva J, Allen TM, Horton H, Venham JE, Rudersdorf RA, Vogel T, Pauza CD, Bontrop RE, DeMars R, Sette A, Hughes AL, Watkins DI (1999b) Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med 5:1270–1276
Evans DT, Jing P, Allen TM, O’Connor DH, Horton H, Venham JE, Piekarczyk M, Dzuris J, Dykhuzen M, Mitchen J, Rudersdorf RA, Pauza CD, Sette A, Bontrop RE, DeMars R, Watkins DI (2000) Definition of five new simian immunodeficiency virus cytotoxic T-lymphocyte epitopes and their restricting major histocompatibility complex class I molecules: evidence for an influence on disease progression. J Virol 74:7400–7410
Furchner M, Erickson AL, Allen T, Watkins DI, Sette A, Johnson PR, Walker CM (1999) The simian immunodeficiency virus envelope glycoprotein contains two epitope presented by the Mamu-A*01 class I molecule. J Virol 73:8035–8039
Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, Heyward WL, Jobes DV, Popovic V, Self SG, Sinangil F, Burke D, Berman PW (2005) Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis 191:666–677
Hirsch VM, Lifson JD (2000) Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv Pharmacol 49:437–477
Horton H, Rehrauer W, Meek EC, Shultz MA, Piekarczyk MS, Jing P, Carter DK, Steffen SR, Calore B, Urvater JA, Vogel TU, Wilson NA, Watkins DI (2001) A common rhesus macaque MHC class I molecule which binds a cytotoxic T-lymphocyte epitope in Nef of simian immunodeficiency virus. Immunogenetics 53:423–426
Kaslow RA, Carrington M, Apple R, Park L, Munoz A, Saah AJ, Goedert JJ, Winkler C, O’Brien SJ, Rinaldo C, Detels R, Blattner W, Phair J, Erlich H, Mann DL (1996) Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med 2:405–411
Kelley J, Walter L, Trowsdale J (2005) Comparative genomics of major histocompatibility complexes Immunogenetics 56:683–695
Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, Rathnavalu P, Moore C, Pfafferott KJ, Hilton L, Zimbwa P, Moore S, Allen T, Brander C, Addo MM, Altfeld M, James I, Mallal S, Bunce M, Barber LD, Szinger J, Day C, Klenerman P, Mullins J, Korber B, Coovadia HM, Walker BD, Goulder PJ (2004) Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432:769–775
Knapp LA, Cadavid LF, Eberle ME, Knechtle SJ, Bontrop RE, Watkins DI (1997a) Identification of new mamu-DRB alleles using DGGE and direct sequencing. Immunogenetics 45:171–179
Knapp LA, Lehmann E, Piekarczyk MS, Urvater JA, Watkins DI (1997b) A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657–661
Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, Desrosiers RC (2006) HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol 7:19–23
Lobashevsky AL, Thomas JM (2000) Six Mamu-A locus alleles defined by polymerase chain reaction sequence specific primer method. Hum Immunol. 61:1013–1020
Loffredo JT, Sidney J, Wojewoda C, Dodds E, Reynolds MR, Napoe G, Mothe BR, O’Connor DH, Wilson NA, Watkins DI, Sette A (2004) Identification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J Immunol 173:5064–5076
Loffredo JT, Sidney J, Piaskowski S, Szymanski A, Furlott J, Rudersdorf R, Reed J, Peters B, Hickman-Miller HD, Bardet W, Rehrauer WM, O’Connor DH, Wilson NA, Hildebrand WH, Sette A, Watkins DI (2005) The high frequency Indian rhesus macaque MHC class I molecule, Mamu-B*01, does not appear to be involved in CD8+ T lymphocyte responses to SIVmac239. J Immunol 175:5986–5997
McNeil AJ, Yap PL, Gore SM, Brettle RP, McColl M, Wyld R, Davidson S, Weightman R, Richardson AM, Robertson JR (1996) Association of HLA types A1-B8-DR3 and B27 with rapid and slow progression of HIV disease. QJM 89:177–185
Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, Hallahan CW, Selig SM, Schwartz D, Sullivan J, Connors M (2000) HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci USA 97:2709–2714
Miller MD, Yamamoto H, Hughes AL, Watkins DI, Letvin NL (1991) Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J Immunol 147:320–329
Mothe BR, Sidney J, Dzuris JL, Liebl ME, Fuenger S, Watkins DI, Sette A (2002) Characterization of the peptide-binding specificity of Mamu-B*17 and identification of Mamu-B*17-restricted epitopes derived from simian immunodeficiency virus proteins. J Immunol 169:210–219
Mothe BR, Weinfurter J, Wang C, Rehrauer W, Wilson N, Allen TM, Allison DB, Watkins DI (2003) Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol 77:2736–2740
Muhl T, Krawczak M, Ten Haaft P, Hunsmann G, Sauermann U (2002) MHC class I alleles influence set-point viral load and survival time in simian immunodeficiency virus-infected rhesus monkeys. J Immunol 169:3438–3446
Nathanson N, Hirsch VM, Mathieson BJ (1999) The role of nonhuman primates in the development of an AIDS vaccine. AIDS 13(Suppl A):S113–S120
Newberg MH, Kuroda MJ, Charini WA, Miura A, Lord CI, Schmitz JE, Gorgone DA, Lifton MA, Kuus-Reichel K, Letvin NL (2002) A simian immunodeficiency virus nef peptide is a dominant cytotoxic T lymphocyte epitope in Indian-origin rhesus monkeys expressing the common MHC class I allele mamu-A*02. Virology 301:365–373
O’Connor DH, Mothe BR, Weinfurter JT, Fuenger S, Rehrauer WM, Jing P, Rudersdorf RR, Liebl ME, Krebs K, Vasquez J, Dodds E, Loffredo J, Martin S, McDermott AB, Allen TM, Wang C, Doxiadis GG, Montefiori DC, Hughes A, Burton DR, Allison DB, Wolinsky SM, Bontrop R, Picker LJ, Watkins DI (2003) Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J Virol 77:9029–9040
Olerup O, Zetterquist H (1991) HLA-DRB1*01 subtyping by allele-specific PCR amplification: a sensitive, specific and rapid technique. Tissue Antigens 37:197–204
Olerup O, Zetterquist H (1992) HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching cadaveric transplantation. Tissue Antigens 39:225–235
Otting N, Heijmans CM, Noort RC, de Groot NG, Doxiadis GG, van Rood JJ, Watkins DI, Bontrop RE (2005) Unparalleled complexity of the MHC class I region in rhesus macaques. Proc Natl Acad Sci USA 102:1626–1631
Robinson J, Waller MJ, Fail SC, Marsh SG (2006) The IMGT/HLA and IPD databases. Hum Mutat 27:1192–1199
Robinson S, Charini WA, Newberg MH, Kuroda MJ, Lord CI, Letvin NL (2001) A commonly recognized simian immunodeficiency virus Nef epitope presented to cytotoxic T lymphocytes of Indian-origin rhesus monkeys by the prevalent major histocompatibility complex class I allele Mamu-A*02. J Virol 75:10179–10186
Schramm RD, Paprocki AM, Watkins D (2002) Birth of MHC-defined rhesus monkeys produced by assisted reproductive technology. Vaccine 20:603–607
Sette A, Sidney J, Bui HH, del Guercio MF, Alexander J, Loffredo J, Watkins DI, Mothe BR (2005) Characterization of the peptide-binding specificity of Mamu-A*11 results in the identification of SIV-derived epitopes and interspecies cross-reactivity. Immunogenetics 57:53–68
Shiina T, Ota M, Shimizu S, Katsuyama Y, Hashimoto N, Takasu M, Anzai T, Kulski JK, Kikkawa E, Naruse T, Kimura N, Yanagiya K, Watanabe A, Hosomichi K, Kohara S, Iwamoto C, Umehara Y, Meyer A, Wanner V, Sano K, Macquin C, Ikeo K, Tokunaga K, Gojobori T, Inoko H, Bahram S (2006) Rapid evolution of major histocompatibility complex class I genes in primates generates new disease alleles in humans via hitchhiking diversity. Genetics 173:1555–1570
Sibal LR, Samson KJ (2001) Nonhuman primates: a critical role in current disease research. ILAR J 42:74–84
Su J, Luscher MA, Xiong Y, Rustam T, Amara RR, Rakasz E, Robinson HL, MacDonald KS (2005) Novel simian immunodeficiency virus CTL epitopes restricted by MHC class I molecule Mamu-B*01 are highly conserved for long term in DNA/MVA-vaccinated, SHIV-challenged rhesus macaques. Int Immunol 17:637–648
Tanaka-Takahashi Y, Yasunami M, Naruse T, Hinohara K, Matano T, Mori K, Miyazawa M, Honda M, Yasutomi Y, Nagai Y, Kimura A (2007) Reference strand-mediated conformation analysis-based typing of multiple alleles in the rhesus macaque MHC class I Mamu-A and Mamu-B loci. Electrophoresis 28:918–924
Vogel TU, Friedrich TC, O’Connor DH, Rehrauer W, Dodds EJ, Hickman H, Hildebrand W, Sidney J, Sette A, Hughes A, Horton H, Vielhuber K, Rudersdorf R, De Souza IP, Reynolds MR, Allen TM, Wilson N, Watkins DI (2002) Escape in one of two cytotoxic T-lymphocyte epitopes bound by a high-frequency major histocompatibility complex class I molecule, Mamu-A*02: a paradigm for virus evolution and persistence? J Virol 76:11623–11636
Voss G, Letvin NL (1996) Definition of human immunodeficiency virus type 1 gp120 and gp41 cytotoxic T-lymphocyte epitopes and their restricting major histocompatibility complex class I alleles in simian-human immunodeficiency virus-infected rhesus monkeys. J Virol 70:7335–7340
Watanabe N, McAdam SN, Boyson JE, Piekarczyk MS, Yasutomi Y, Watkins DI, Letvin NL (1994) A simian immunodeficiency virus envelope V3 cytotoxic T-lymphocyte epitope in rhesus monkeys and its restricting major histocompatibility complex class I molecule Mamu-A*02. J Virol 68:6690–6696
Watkins DI, Kannagi M, Stone ME, Letvin NL (1988) Major histocompatibility complex class I molecules of nonhuman primates. Eur J Immunol 18:1425–1432
Welsh K, Bunce M (1999) Molecular typing for the MHC with PCR-SSP. Rev Immunogenet 1:157–176
Wojcechowskyj JA, Yant LJ, Wiseman RW, O’Connor SL, O’Connor DH (2007) Control of simian immunodeficiency virus SIVmac239 is not predicted by inheritance of Mamu-B*17-containing haplotypes. J Virol 81:406–410
Yant LJ, Friedrich TC, Johnson RC, May GE, Maness NJ, Enz AM, Lifson JD, O’Connor DH, Carrington M, Watkins DI (2006) The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol 80:5074–5077 (Erratum in: J Virol 2006 80:6720)
Yasutomi Y, McAdam SN, Boyson JE, Piekarczyk MS, Watkins DI, Letvin NL (1995) A MHC class I B locus allele-restricted simian immunodeficiency virus envelope CTL epitope in rhesus monkeys. J Immunol 154:2516–2522
Zhang ZQ, Fu TM, Casimiro DR, Davies ME, Liang X, Schleif WA, Handt L, Tussey L, Chen M, Tang A, Wilson KA, Trigona WL, Freed DC, Tan CY, Horton M, Emini EA, Shiver JW (2002) Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. J Virol 76:12845–12854
Acknowledgments
We express our gratitude to Jess Maxwell, Tim Jacoby, Julie Leblanc, Brock Christiansen, Millicent Schultz, Elizabeth Meek, and Christopher Murvine as previous members of the laboratory for their extensive assistance to the MHC class I PCR-SSP typing project and gratefully recognize Rudy Rudersdorf and Lyle Wallace for their significant contributions of transfectant cell lines and MHC class I cDNA derived sequences. We acknowledge Nancy A. Wilson, David O’Connor, Thomas C. Friedrich, Adrian McDermott, and Roger Wiseman for their insightful discussions. We also collectively thank the Immunology, Virology, and DNA Sequencing Core Laboratories at the National Primate Research Center, University of Wisconsin–Madison for their technical assistance. We appreciate Amanda Espinosa for her help and administrative contributions to our laboratory. Finally, we value the opportunity to serve as a MHC typing service for numerous investigators, without their interest and support this work would not have been possible.
This research was supported by National Institutes of Health (NIH) grant R24 RR016038 and NIAID contract no. HHSN266200400088C. This publication was also made possible in part by Grant Number P51 RR000167 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), to the Wisconsin National Primate Research Center, University of Wisconsin–Madison. This research was conducted at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaizu, M., Borchardt, G.J., Glidden, C.E. et al. Molecular typing of major histocompatibility complex class I alleles in the Indian rhesus macaque which restrict SIV CD8+ T cell epitopes. Immunogenetics 59, 693–703 (2007). https://doi.org/10.1007/s00251-007-0233-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-007-0233-7