Abstract
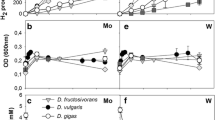
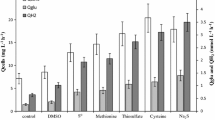
Biological sulfate (SO4) reduction with carbon monoxide (CO) as electron donor was investigated. Four thermophilic SO4-reducing bacteria, Desulfotomaculum thermoacetoxidans (DSM 5813), Thermodesulfovibrio yellowstonii (ATCC 51303), Desulfotomaculum kuznetsovii (DSM 6115; VKM B-1805), and Desulfotomaculum thermobenzoicum subsp. thermosyntrophicum (DSM 14055), were studied in pure culture and in co-culture with the thermophilic carboxydotrophic bacterium Carboxydothermus hydrogenoformans (DSM 6008). D. thermoacetoxidans and T. yellowstonii were extremely sensitive to CO: their growth on pyruvate was completely inhibited at CO concentrations above 2% in the gas phase. D. kuznetsovii and D. thermobenzoicum subsp. thermosyntrophicum were less sensitive to CO. In pure culture, D. kuznetsovii and D. thermobenzoicum subsp. thermosyntrophicum were able to grow on CO as the only electron donor and, in particular in the presence of hydrogen/carbon dioxide, at CO concentrations as high as 50–70%. The latter SO4 reducers coupled CO oxidation to SO4 reduction, but a large part of the CO was converted to acetate. In co-culture with C. hydrogenoformans, D. kuznetsovii and D. thermobenzoicum subsp. thermosyntrophicum could even grow with 100% CO (PCO=120 kPa).


Similar content being viewed by others
References
Adams MWW (1990) The structure and mechanism of iron-hydrogenases. Biochim Biophys Acta 1020:115–145
Bennett B, Lemon BJ, Peters W (2000) Reversible carbon monoxide binding and inhibition at the active site of Fe-only hydrogenase. Biochemistry 39:7455–7460
Berlier Y, Fauque GD, LeGall J, Choi ES, Peck HD Jr, Lespinat PA (1987) Inhibition studies of three classes of Desulfovibrio hydrogenases: application to the further characterization of the multiple hydrogenases found in Desulfovibrio vulgaris Hilderborough. Biochem Biophys Res Commun 146:147–153
Daniels LG, Fuchs G, Thauer RK, Zeikus JG (1977) Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol 132:118–126
Davidova MN, Tarasova NB, Mukhitova FK, Karpilova IU (1994) Carbon monoxide in metabolism of anaerobic bacteria. Can J Microbiol 40:417–425
Fardeau M-L, Salinas MB, L’Haridon S, Jeanthon Ch, Verhe F, Cayol J-L, Patel BKC, Garcia J-L, Ollivier B (2004) Isolation from oil reservoirs of novel thermophilic anaerobes phylogenetically related to Thermoanaerobacter subterraneus: reassignment T. subterraneus, Thermoanaerobacter yonseiensis, Thermoanaerobacter tengcongensis and Carboxydibrachium pacificum, to Caldanaerobacter subterraneus gen. nov., sp. nov., comb. nov. as four novel subspecies. Int J Syst Evol Microbiol 54:467–474
Graboski MS (1984) The production of synthesis gas from methane, coal and biomass. In: Herman RG (ed) Catalytic conversion of synthesis gas and alcohols to chemicals. Plenum, New York, pp 37–52
Henry EA, Devereux R, Maki JS, Gilmour CC, Woese CR, Mandelco L, Schauder R, Remsen CC, Mitchell R (1994) Characterization of a new thermophilic sulfate-reducing bacterium Thermodesulfovibrio yellowstonii, gen. nov. and sp. nov.: its phylogenetic relationship to Thermodesulfobacterium commune and their origins deep within the bacterial domain. Arch Microbiol 161:62–69
Karpilova IYu, Davidova MN, Belyaeva MI (1983) The effect of carbon monoxide on the growth and CO oxidation in sulfate-reducing bacteria (in Russian). Biol Nauki 1:85–88
Klemps R, Cypionka H, Widdel F, Pfennig N (1985) Growth with hydrogen, and further physiological characteristics of Desulfotomaculum species. Arch Microbiol 143:203–208
Lens PNL, Visser A, Janssen AJH, Hulshoff Pol LW, Lettinga G (1998) Biotechnological treatment of sulfate-rich wastewaters. Crit Rev Environ Sci Technol 28:41–88
Lupton FS, Conrad R, Zeikus JG (1984) CO metabolism of Desulfovibrio vulgaris strain Madison: physiological function in the absence or presence of exogenous substrates. FEMS Microbiol Lett 23:263–268
Maree JP, Gerber A, Hill E (1987) An integrated process for biological treatment of sulphate-containing industrial influents. J Water Pollut Control Fed 59:1069–1074
Min H, Zinder SH (1990) Isolation and characterization of a thermophilic sulfate-reducing bacterium Desulfotomaculum thermoacetoxidans sp. nov. Arch Microbiol 153:399–404
Mörsdorf G, Frunzke K, Gadkari D, Meyer O (1992) Microbial growth on carbon monoxide. Biodegradation 3:61–82
Nazina TN, Ivanova AE, Kunchaveli LP, Rozanova EP (1988) A new sporeforming thermophilic methylotrophic sulfate- reducing bacterium, Desulfotomaculum kuznetsovii sp nov. Mikrobiologiya 57:823–827
Pankhania IP, Gow LA, Hamilton WA (1986) The effect of hydrogen on the growth of Desulfovibrio vulgaris (Hildenborough) on lactate. J Gen Microbiol 132:3349–3356
Perry RH, Green DW, Maloney JO (1997) Perry’s chemical engineers’ handbook, 7th edn. McGraw-Hill, New York, pp 13–25
Plugge C, Balk M, Stams AJM (2002) Desulfotomaculum thermobenzoicum subsp. thermosyntrophicum subsp. nov., a thermophilic syntrophic propionate-oxidizing spore-forming bacterium. Int J Syst Evol Microbiol 52:391–399
Rother M, Metcalf W (2004) Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: an unusual way of life for a methanogenic archaeon. PNAS 101:16929–16934
Sharak Genthner BR, Bryant MP (1982) Growth of Eubacterium limosum with carbon monoxide as the energy source. Appl Environ Microbiol 43:70–74
Sipma J, Lens PNL, Stams AJM, Lettinga G (2003) Carbon monoxide conversion by anaerobic bioreactor sludges. FEMS Microbiol Ecol 44:271–277
Sipma J, Meulepas RJW, Parshina SN, Stams AJM, Lettinga G, Lens PNL (2004) Effect of carbon monoxide, hydrogen and sulfate on thermophilic (55°C) hydrogenic carbon monoxide conversion in two anaerobic bioreactor sludges. Appl Microbiol Biotechnol 64:421–428
Sokolova TG, Gonzalez JM, Kostrikina NA, Chernyh NA, Tourova TP, Kato C, Bonch-Osmolovskaya EA, Robb FT (2001) Carboxydibrachium pacificum gen.nov., sp. nov., a new anaerobic, thermophilic, CO-utilizing marine bacterium from Okinawa Trough. Int J Syst Evol Microbiol 51:141–149
Sokolova TG, Kostrikina NA, Chernyh NA, Tourova TP, Kolganova TV, Bonch-Osmolovskaya EA (2002) Carboxydocella thermoautotrophica gen. nov. sp. nov., a novel anaerobic, CO-utilizing thermophile from a Kamchatkan hot spring. Int J Syst Evol Microbiol 52:1–6
Sokolova TG, Jeanthon Ch, Kostrikina NA, Chernyh NA, Lebedinsky AV, Stackebrandt E, Bonch-Osmolovskaya EA (2004a) The first evidence of anaerobic CO oxidation coupled with H2 production by a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Extremophiles 8:317–323
Sokolova TG, Gonzalez JM, Kostrikina NA, Chernyh NA, Slepova TV, Bonch-Osmolovskaya EA, Robb FT (2004b) Thermosinus carboxydivorans gen. nov., sp. nov., a new anaerobic, thermophilic, carbon-monoxide-oxidizing, hydrogenogenic bacterium from a hot pool of Yellowstone National Park. Int J Syst Evol Microbiol 54:2353–2359
Stams AJM, van Dijk JB, Dijkema C, Plugge CM (1993) Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl Environ Microbiol 59:1114–1119
Svetlichniy VA, Sokolova TA, Gerhardt M, Ringpfel M, Kostrikina NA, Zavarzin GA (1991) Carboxydothermus hydrogenoformans gen. nov., sp. nov. a CO utilizing thermophilic anaerobic bacterium from hydrothermal environments of Kunashir island. Syst Appl Microbiol 14:254–260
Svetlichnyi VA, Sokolova TG, Kostrikina NA, Lysenko AM (1994) A new thermophilic anaerobic carboxydotrophic bacterium Carboxydothermus restrictus sp. nov. Microbiology 63:294–297
Trüper HG, Schlegel HG (1964) Sulfur metabolism in Thiorhodaceae. 1.Quantitative measurement of growing cells of Chromatium okenii. Antonie Van Leeuwenhoek 30:225–238
Houten RT van, HulshoffPol LW, Lettinga G (1994) Biological sulphate reduction using gas-lift reactors fed with hydrogen and carbon dioxide as energy and carbon source. Biotechnol Bioeng 44:586–594
Houten RT van, van der Spoel H, van Aelst AC, Hulshoff Pol LW, Lettinga G (1996) Biological sulphate reduction using synthesis gas as energy and carbon source. Biotechnol Bioeng 50:136–144
Houten RT van, Yu Yun S, Lettinga G (1997) Thermophilic sulphate and sulphite reduction in lab-scale gas-lift reactors using H2/CO2 as energy and carbon source. Biotechnol Bioeng 55:807–814
Widdel F, Hansen TA (1992) The dissimilatory sulfate- and sulfur-reducing bacteria. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer K-H (eds) The prokaryotes, 2nd edn. Springer, Berlin Heidelberg New York, pp 583–624
Acknowledgements
This research was financially supported by Shell Global Solutions, Paques Natural Solutions B.V., the Technology Foundation (STW) and the Earth and Life Sciences Foundation (ALW) of the Netherlands Organization of Scientific Research (NWO) (The Netherlands).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parshina, S.N., Kijlstra, S., Henstra, A.M. et al. Carbon monoxide conversion by thermophilic sulfate-reducing bacteria in pure culture and in co-culture with Carboxydothermus hydrogenoformans. Appl Microbiol Biotechnol 68, 390–396 (2005). https://doi.org/10.1007/s00253-004-1878-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1878-x