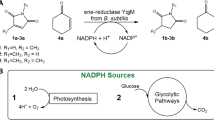
Abstract
The obligate photoautotrophic cyanobacterium Synechococcus PCC7942 and the photoheterotrophic heterocystous cyanobacterium Noctoc muscorum are able to reduce prochiral ketones asymmetrically to optical pure chiral alcohols without light. An example is the synthesis of S-pentafluoro(phenyl-)ethanol with an enantiomeric excess >99% if 2′-3′-4′-5′-6′-pentafluoroacetophenone is used as substrate. If no light is available for regeneration of the cofactor nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH), glucose is used as cosubstrate. Membrane disintegration during asymmetric reduction promotes cytosolic energy generating metabolic pathways. Observed regulatory effects depicted by an adenosine triphosphate (ATP) to nicotinamide adenine dinucleotide phosphate (oxidized form) (NADP+) ratio of 3:1 for efficient cofactor recycling indicate a metabolization via glycolisis. The stoichiometric formation of the by-product acetate (1 mol acetate/1 mol chiral alcohol) indicates homoacetic acid fermentation for cofactor regeneration including the obligate photoautotrophic cyanobacterium Synechococcus PCC7942.






Similar content being viewed by others
References
Allen MM (1968) Simple conditions for the growth of unicellular blue-green algae on plates. J Phycol 4:1–4
Carr NG, Whitton BA (1973) The biology of blue-green algae. Botanical monographs. Blackwell, Osney Mead, Oxford
Eckstein M, Daußmann T, Kragl U (2004) Recent developments in NAD(P)H regeneration for enzymatic reductions in one- and two-phase systems. Biocatal Biotransform 22:89–96
Ferrer M, Golyshina OV, Chernikova TN, Khachane AN, Martins dos Santos VAP, Yakimov MM, Timmis KN, Golyshin PN (2005) Microbial enzymes mined from the urania deep-sea hypersaline anoxic basin. Chem Biol 12:904–985
Franco-Lara E, Havel J, Peterat F, Weuster-Botz D (2006) Model-supported optimization of phototrophic growth in a stirred-tank photobioreactor. Biotechnol Bioeng 95(6):1177–1187
Gelo-Pujic M, Le Gyader F, Schlama T (2006) Microbial and homogenous asymmetric catalysis in the reduction of 1-[3,5-bis(trifluoromethyl)phenyl]ethanol. Tetrahedron: Asymmetry 17:2000–2005
Hamada H, Kondo Y, Ishihara K, Nakajima N, Hamada H, Kurihara R, Hirata T (2003) Stereoselective biotransformation of limonene and limonene oxide by cyanobacterium, Synechococcus sp. PCC 7942. J Biosci Bioeng 96(6):581–584
Havel J, Weuster-Botz D (2006) Comparative study of cyanobacteria as biocatalysts for the asymmetric synthesis of chiral building blocks. Eng Life Sci 6:175–179
Havel J, Link H, Hofinger M, Franco-Lara E, Weuster-Botz D (2006) Comparison of genetic algorithms for experimental multi-objective optimization on the example of medium design for cyanobacteria. Biotechnol J 1:549–555
Heiss C, Laivenieks M, Zeikus G, Phillips RS (2001) Mutation of cysteine-295 to alanine in secondary alcohol dehydrogenase from Thermoanaerobacter ethanolicus affects enantioselectivity and substrate specificity of ketone reductions. Bioorganic Med Chem 9:1659–1666
Hummel W, Abokitse K, Drauz K, Rollmann C, Gröger H (2003) Towards a large-scale asymmetric reduction process with isolated enzymes: expression of an (S)-alcohol dehydrogenase in E. coli and studies on the synthetic potential of this biocatalyst. Adv Synth Catal 345:153–159
Knowles VL, Plaxton WC (2003) From genome to enzyme: analysis of key glycolitic and oxidative pentosephosphate pathway enzymes in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 44(7):758–763
Kroutil W, Mang H, Edegger K, Faber K (2004) Recent advances in the biocatalytic reduction of ketones and oxidation o sec-alcohols. Curr Opin Chem Biol 8:120–126
Nakamura K, Yamanaka R (2002) Light-mediated regulation of asymmetric reduction of ketones by a cyanobacterium. Tetrahedron: Asymmetry 16:2529–2533
Nakamura K, Yamanaka R, Tohi K, Hamada H (2000) Cyanobacterium-catalyzed asymmetric reduction of ketones. Tetrahedron Lett 41:6799–6802
Nakamura K, Yamanaka R, Matsuda T, Harada T (2003) Recent developments in asymmetric reduction of ketones with biocatalysts. Tetrahedron: Asymmetry 14(18):2659–2681
Noma Y, Asakawa Y (1992) Enantio- and stereoselectivity in the biotransformation of carveols by Euglena gracilis Z. Phytochemistry 31(6):2009–2011
Pearce J, Carr NG (1969) The incorporation and metabolism of glucose by Anabaena variabilis. J Gen Microbiol 54:451–462
Peschek GA (1979) The role of the calvin cycle for anoxygenic CO2 photoassimilation in Anacystic nidulans. FEBS Lett 106(1):34–38
Pfruender H, Jones R, Weuster-Botz D (2006) Water immiscible ionic liquids as solvents for whole cell biocatalysis. J Biotechnol 124:182–190
Reetz MT (2006) Direct evolution of enantioselective enzymes as catalysts for organic synthesis. Adv Catal 49:1–69
Rippka R, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111(2):1–61
Rosen TC, Feldmann R, Dünkelmann P, Daußmann T (2006) Bioreductive synthesis of perfluorinated chiral alcohols. Tetrahedron Lett 47:4803–4806
Shimoda K, Hirata T (2000) Biotransformation of enones with biocatalysts- two enone reductases from Astasia longa. J Mol Catal B Enzym 8:255–264
Shimoda K, Kubota N, Hamada H, Kajib M, Hirata T (2004) Asymmetric reduction of enones with Synechococcus sp. PCC 7942. Tetrahedron: Asymmetry 15:1677–1679
Stal LJ (1992) Poly(hydroxyalkanoate) in cyanobacteria: an overview. FEMS Microbiol Rev 103:169–180
Stal LJ, Moezelaar R (1997) Fermentation in cyanobacteria. FEMS Microbiol Rev 21:179–211
Wu JT, Wu LH, Knight JA (1986) Stability of NADPH: effects of various factors on the kinetics of degradation. Clin Chem 32:314–319
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Havel, J., Weuster-Botz, D. Cofactor regeneration in phototrophic cyanobacteria applied for asymmetric reduction of ketones. Appl Microbiol Biotechnol 75, 1031–1037 (2007). https://doi.org/10.1007/s00253-007-0910-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-0910-3