Abstract
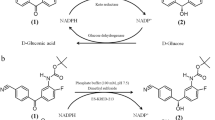
Enzymes are able to perform reactions under mild conditions, e.g., pH and temperature, with remarkable chemo-, regio-, and stereoselectivity. Because of this feature, the number of biocatalysts used in organic synthesis has rapidly increased during the last decades, especially for the production of chiral compounds. The present review highlights biotechnological processes for the production of chiral alcohols by reducing prochiral ketones. These reactions can be catalyzed by either isolated enzymes or whole cells that exhibit ketone-reducing activity. The use of isolated enzymes is often preferred because of a higher volumetric productivity and the absence of side reactions. Both types of catalysts have also deficiencies limiting their use in synthesis of chiral alcohols. Because reductase-catalyzed reactions are dependent on cofactors, one major task in process development is to provide an effective method for regeneration of the consumed cofactors. In this paper, strategies for cofactor regeneration in biocatalytic ketone reduction are reviewed. Furthermore, different processes carried out on laboratory and industrial scales using isolated enzymes are presented. Attention is turned to process parameters, e.g., conversion, yield, enantiomeric excess, and process strategies, e.g., the application of biphasic systems or methods of in situ (co)product recovery. The biocatalytic production of chiral alcohols utilizing whole cells is presented in part II of this review (Goldberg et al., Appl Microbiol Biotechnol, 2007).










Similar content being viewed by others
References
Abril O, Whitesides GM (1982) Hybrid organometallic/enzymatic catalyst systems: regeneration of NADH using dihydrogen. J Am Chem Soc 104:1552–1554
Adam W, Lazarus M, Saha-Möller CR, Schreier P (1999) Biocatalytic synthesis of optically active α-oxyfunctionalized carbonyl compounds. Acc Chem Res 32:837–845
Avi M, Fechter MH, Gruber K, Belaj F, Pöchlauer P, Griengl H (2004) Hydroxynitrile lyase catalyzed synthesis of heterocyclic (R)- and (S)-cyanohydrins. Tetrahedron 60:10411–10418
Bhaduri S, Mathur P, Payra P, Sharma K (1998) Coupling of catalyses by carbonyl clusters and dehydrogenases: reduction of pyruvate to l-lactate by dihydrogen. J Am Chem Soc 120:12127–12128
Biade AE, Bourdillon C, Lava JM, Mairesse G, Moiroux J (1992) Complete conversion of l-lactate into d-lactate. A generic approach involving enzymatic catalysis, electrochemical oxidation of NADH, and electrochemical reduction of pyruvate. J Am Chem Soc 114:893–897
Blaser HU, Malan C, Pugin B, Spindler F, Steiner H, Studer M (2003) Selective hydrogenation for fine chemicals: recent trends and new developments. Adv Synth Catal 345:103–151
Bommarius AS, Schwarm M, Stingl K, Kottenhahn M, Huthmacher K, Drauz K (1995) Synthesis and use of enantiomerically pure tert-leucine. Tetrahedron Asymmetry 6:2851–2888
Bornscheuer U, Reif OW, Lausch R, Freitag R, Scheper T, Kolisis FN, Menge U (1994) Lipase of Pseudomonas cepacia for biotechnological purposes: purification, crystallization and characterization. Biochim Biophys Acta 1201:55–60
Bortolini OMS (1997) An easy approach to the synthesis of optically active vic-diols: a new single-enzyme system. J Org Chem 62:1854–1856
Breuer M, Ditrich K, Habicher T, Hauer B, Keßeler M, Stürmer R, Zelinski T (2004) Industrielle Verfahren zur Herstellung von optisch aktiven Zwischenprodukten. Angew Chem 116:806–843 (also in Angew Chem Int Ed 43:788–824)
Buchholz S, Gröger H (2006) Enantioselective biocatalytic reduction of ketones for the synthesis of optically active alcohols. In: Patel RN (ed) Biocatalysis in the pharmaceutical and biotechnology industries. Taylor & Francis, New York, pp 757–790
Buque-Taboada EM, Straathof AJJ, Heijnen JJ, van der Wielen LAM (2006) In situ product recovery (ISPR) by crystallization: basic principles, design, and potential applications in whole-cell biocatalysis. Appl Microbiol Biotechnol 71:1–12
Choudary BM, Chowdari NS, Madhi S, Kantam ML (2001) A trifunctional catalyst for the synthesis of chiral diols. Angew Chem 113:4755–4759 (also in Angew Chem Int Ed 40:4655–4759)
Costas AMG, White AK, Metcalf WW (2001) Purification and characterization of a novel phosphorus-oxidizing enzyme from Pseudomonas stutzeri WM88. J Biol Chem 276:17429–17436
Daußmann T, Hennemann HG, Rosen TC (2006a) Enzymatische technologien zur synthese chiraler alkohol-derivate. Chem Ing Tech 78:249–255
Daußmann T, Dünkelmann P, Lütz S (2006b) Chiral alcohols. CHEManager (Europe) 3:8
Daußmann T, Rosen TC, Dünkelmann P (2006c) Oxidoreductases and hydroxynitrilase lyases: complementary enzymatic technologies for chiral alcohols. Eng Life Sci 6:125–129
Davis C, Grate J, Gray D, Gruber J, Huismann G, Ma S, Newman L, Sheldon R (2005) Enzymatic processes for the production of 4-substituted 3-hydroxybutyric acid derivatives. Codexis, Patent no. WO04015132
Degenring D, Schröder I, Wandrey C, Liese A, Greiner L (2004) Resolution of 1,2-diols by enzyme-catalyzed oxidation with anodic, mediated cofactor regeneration in the extractive membrane reactor: gaining insight by adaptive simulation. Org Process Res Dev 8:213–218
Demir AS, Şeşenoglu Ö, Eren E, Hosrik B, Pohl M, Janzen E, Kolter D, Feldmann R, Dünkelmann P, Müller M (2002) Enantioselective synthesis of α-hydroxy ketones via benzaldehyde lyase-catalyzed C–C bond formation reaction. Adv Synth Catal 344:96–103
Detry J, Rosenbaum T, Lütz S, Hahn D, Jaeger KE, Müller M, Eggert T (2006) Biocatalytic production of enantiopure cyclohexane-trans-1,2-diol using extracellular lipases from Bacillus subtilis. Appl Microbiol Biotechnol 72:1107–1116
Domínguez de María P, Stillger T, Pohl M, Wallert S, Drauz K, Groger H, Trauthwein H, Liese A (2006) Preparative enantioselective synthesis of benzoins and (R)-2-hydroxy-1-phenylpropanone using benzaldehyde lyase. J Mol Catal B Enzym 38:43–47
Eckstein M, Villela M, Liese A, Kragl U (2004) Use of an ionic liquid in a two-phase system to improve an alcohol dehydrogenase catalysed reduction. Chem Commun 9:1084–1085
Elend C, Schmeisser C, Leggewie C, Babiak P, Carballeira JD, Steele HL, Reymond JL, Jaeger KE, Streit WR (2006) Isolation and biochemical characterization of two novel metagenome-derived esterases. Appl Environ Microbiol 72:3637–3645
Ernst M, Kaup B, Müller M, Bringer-Meyer S, Sahm H (2005) Enantioselective reduction of carbonyl compounds by whole-cell biotransformation, combining a formate dehydrogenase and a (R)-specific alcohol dehydrogenase. Appl Microbiol Biotechnol 66:629–634
Faber K (2004) Biotransformations in organic chemistry (5th edn.). Springer, Berlin
Fechter MH, Griengl H (2004) Hydroxynitrile lyases: biological sources and application as biocatalysts. Food Technol Biotechnol 42:287–294
Ferloni C, Heinemann M, Hummel W, Daußmann T, Büchs J (2004) Optimization of enzymatic gas-phase reactions by increasing the long-term stability of the catalyst. Biotechnol Prog 20:975–978
Findrik Z, Vasic-Racki D, Lütz S, Daußmann T, Wandrey C (2005) Kinetic modelling of acetophenone reduction catalyzed by alcohol dehydrogenase from Thermoanaerobacter sp. Biotechnol Lett 27:1087–1095
Gaisberger RP, Fechter MH, Griengl H (2004) The first hydroxynitrile lyase catalysed cyanohydrin formation in ionic liquids. Tetrahedron Asymmetry 15:2959–2963
Goldberg K, Edegger K, Kroutil W, Liese A (2006) Overcoming the thermodynamic limitation in asymmetric hydrogen transfer reactions catalyzed by whole cells. Biotechnol Bioeng 95:192–198
Goldberg K, Schroer K, Lütz S, Liese A (2007) Biocatalytic ketone reduction—a powerful tool for the production of chiral alcohols—part II: whole cell reductions. Appl Microbiol Biotechnol (in this issue)
Greiner L, Müller DH, van den Ban ECD, Wöltinger J, Wandrey C, Liese A (2003) Membrane aerated hydrogenation: enzymatic and chemical homogeneous catalysis. Adv Synth Catal 345:679–683
Griengl H, Hickel A, Johnson DV, Kratky C, Schmidt M, Schwab H (1997) Enzymatic cleavage and formation of cyanohydrins: a reaction of biological and synthetic relevance. Chem Commun 1933–1940
Haberland J, Hummel W, Daußmann T, Liese A (2002) New continuous production process for enantiopure (2R,5R)-hexanediol. Org Process Res Dev 6:458–462
Hildebrand F, Lütz S (2006) Immobilisation of alcohol dehydrogenase from Lactobacillus brevis and its application in a plug-flow reactor. Tetrahedron Asymmetry 17:3219–3225
Hildebrand F, Kühl S, Pohl M, Vasic-Racki D, Müller M, Wandrey C, Lütz S (2006) The production of (R)-2-hydroxy-1-phenyl-propan-1-one derivatives by benzaldehyde lyase from Pseudomonas fluorescens in a continuously operated membrane reactor. Biotechnol Bioeng 96(5):835–843
Hilt G, Jarbawi T, Heineman WR, Steckhan E (1997) An analytical study of the redox behavior of 1,10-phenanthroline-5,6-dione, its transition-metal complexes, and its N-monomethylated derivative with regard to their efficiency as mediators of NAD(P)+ regeneration. Chem Eur 3:79–88
Hollmann F, Schmid A (2004) Electrochemical regeneration of oxidoreductases for cell-free biocatalytic redox reactions. Biocatal Biotransform 22:63–88
Hollmann F, Kleeb A, Otto K, Schmid A (2006) Corrigendum to “Coupled chemoenzymatic transfer hydrogenation catalysis for enantioselective reduction and oxidation reactions”. Tetrahedron Asymmetry 17:867–868
Honda K, Ishige T, Kataoka M, Shimizu S (2006) Microbial and enzymatic processes for the production of chiral compounds. In: Patel RN (ed) Biocatalysis in the pharmaceutical and biotechnology industries. Taylor & Francis, New York, pp 529–546
Hummel W (1997) New alcohol dehydrogenases for the synthesis of chiral compounds. Adv Biochem Eng Biotechnol 58:145–184
Hummel W, Kula MR (1989) Dehydrogenases for the synthesis of chiral compounds. Eur J Biochem 184:1–13
Hummel W, Abokitse K, Drauz K, Rollmann C, Gröger H (2003) Towards a large-scale asymmetric reduction process with isolated enzymes: expression of an (S)-alcohol dehydrogenase in E. coli and studies on the synthetic potential of this biocatalyst. Adv Synth Catal 345:153–159
Jaeger KE, Eggert T (2002) Lipases for biotechnology. Curr Opin Biotechnol 13:390–397
Jaeger KE, Schneidinger B, Rosenau F, Werner M, Lang D, Dijkstra BW, Schimossek K, Zonta A, Reetz MT (1997) Bacterial lipases for biotechnological applications. J Mol Catal B Enzym 3:3–12
Johannes TW, Woodyer RD, Zhao H (2007) Efficient regeneration of NADPH using an engineered phosphite dehydrogenase. Biotechnol Bioeng 96:18–26
Jones JB, Sneddon DW, Higgins W, Lewis A (1972) Preparative-scale reductions of cyclic ketone and aldehyde substrates of horse liver alcohol dehydrogenase with in situ sodium dithionite recycling of catalytic amounts of NAD. J Chem Soc Chem Commun 1972:856–857
Julliard M, Le Petit J, Ritz P (2004) Regeneration of NAD+ cofactor by photosensitized electron transfer in an immobilized alcohol dehydrogenase system. Biotechnol Bioeng 28:1774–1779
Kataoka M, Rohani LPS, Wada M, Kita K, Yanase H, Urabe I, Shimizu S (1998) Escherichia coli transformant expressing the glucose dehydrogenase gene from Bacillus megaterium as a cofactor regenerator in a chiral alcohol production system. Biosci Biotechnol Biochem 62:167–169
Kihumbu D, Stillger T, Hummel W, Liese A (2002) Enzymatic synthesis of all stereoisomers of 1-phenylpropane-1,2-diol. Tetrahedron Asymmetry 13:1069–1072
Kim MJ, Whitesides GM (1988) l-Lactate dehydrogenase: substrate specificity and use as a catalyst in the synthesis of homochiral 2-hydroxy acids. J Am Chem Soc 110:2959–2964
Kizaki N, Yasohara Y, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Synthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55:590–595
Klibanov AM, Puglisi AV (1980) The regeneration of coenzymes using immobilized hydrogenase. Biotechnol Lett 2:445–450
Koike T, Murata K, Ikariya T (2000) Stereoselective synthesis of optically active α-hydroxy ketones and anti-1,2-diols via asymmetric transfer hydrogenation of unsymmetrically substituted 1,2-diketones. Org Lett 2:3833–3836
Kosjek B, Stampfer W, Pogorevc M, Goessler W, Faber K, Kroutil W (2004) Purification and characterization of a chemotolerant alcohol dehydrogenase applicable to coupled redox reactions. Biotechnol Bioeng 86:55–62
Kragl U, Eckstein M, Kaftzik N (2002) Enzyme catalysis in ionic liquids. Curr Opin Biotechnol 13:565–571
Kruse W, Hummel W, Kragl U (1996) Alcohol-dehydrogenase-catalyzed production of chiral hydrophobic alcohols. A new approach leading to a nearly waste-free process. Recl Trav Chim Pays-Bas 115:239–243
Kula MR, Kragl U (2000) In: Patel RN (ed) Stereoselective biocatalysis. Marcel Dekker, New York
Kurlemann N, Liese A (2004) Immobilization of benzaldehyde lyase and its application as a heterogeneous catalyst in the continuous synthesis of a chiral 2-hydroxy ketone. Tetrahedron Asymmetry 15:2955–2958
Lamare S, Legoy MD, Graber M (2004) Solid/gas bioreactors: powerful tools for fundamental research and efficient technology for industrial applications. Green Chem 6:445–458
Leksawasdi N, Chow YYS, Breuer M, Hauer B, Rosche B, Rogers PL (2004) Kinetic analysis and modelling of enzymatic (R)-phenylacetylcarbinol batch biotransformation process. J Biotechnol 111:179–189
Leonida MD (2001) Redox enzymes used in chiral syntheses coupled to coenzyme regeneration. Curr Med Chem 8:345–369
Liese A, Karutz M, Kamphuis J, Wandrey C, Kragl U (1996) Enzymatic resolution of 1-phenyl-1,2-ethanediol by enantioselective oxidation: overcoming product inhibition by continuous extraction. Biotechnol Bioeng 51:544–550
Liese A, Zelinski T, Kula MR, Kierkels H, Karutz M, Kragl U, Wandrey C (1998) A novel reactor concept for the enzymatic reduction of poorly soluble ketones. J Mol Catal B Enzym 4:91–99
Liese A, Seelbach C, Wandrey C (2006) Industrial biotransformations (2nd edn.). GmbH, Weinheim
Liu J, Hsu CC, Wong CH (2004) Sequential aldol condensation catalyzed by DERA mutant Ser238Asp and a formal total synthesis of atorvastatin. Tetrahedron Lett 45:2439–2441
Lütz S (2006) Transition metal catalyzed regeneration of nicotineamide cofactors. In: de Vries JG, Elsevier CJ (eds) The handbook of homogeneous hydrogenation (vol III). GmbH, Weinheim, pp 1471–1482
Lye GJ, Woodley JM (1999) Application of in situ product-removal techniques to biocatalytic processes. Trends Biotechnol 17:395–402
Makino Y, Ding JY, Negoro S, Urabe I, Okada H (1989) Purification and characterization of a new glucose dehydrogenase from vegetative cells of Bacillus megaterium. J Ferment Bioeng 67:374–379
McWhirter RB, Klapper MH (1990) Semiquinone radicals of methylamine dehydrogenase, methoxatin, and related o-quinones: a pulse radiolysis study. Biochemistry 29:6919–6926
Mertens R, Liese A (2004) Biotechnological application of hydrogenases. Curr Opin Biotechnol 15:343–348
Mertens R, Greiner L, van den Ban ECD, Haaker HBCM, Liese A (2003) Practical applications of hydrogenase I from Pyrococcus furiosus for NADPH generation and regeneration. J Mol Catal B Enzym 24–25:39–52
Mochizuki N, Hiramatsu S, Sugai T, Ohta H, Morita H, Itokawa H (1995) Improved conditions for the production and characterization of 1-arylpropane-1,2-diols and related compounds. Biosci Biotechnol Biochem 59:2282–2291
Müller M (2005) Chemoenzymatische synthese von bausteinen für Statin-Seitenketten. Angew Chem 117:366–369 (also in Angew Chem Int Ed 44:362–365)
Neuhauser W, Steininger M, Haltrich D, Kulbe KD, Nidetzky B (1998) A pH-controlled fed-batch process can overcome inhibition by formate in NADH-dependent enzymatic reductions using formate dehydrogenase-catalyzed coenzyme regeneration. Biotechnol Bioeng 60:278–282
Nidetzky B, Neuhauser W, Haltrich D, Kulbe KD (1996) Continuous enzymatic production of xylitol with simultaneous coenzyme regeneration in a charged membrane reactor. Biotechnol Bioeng 52:387–396
Orlich B, Berger H, Lade M, Schomacker R (2000) Stability and activity of alcohol dehydrogenases in W/O-emulsions: enantioselective reduction including cofactor regeneration. Biotechnol Bioeng 70:638–646
Patel RN, Banerjee A, McNamee CG, Brzozowski D, Hanson RL, Szarka LJ (1993) Enantioselective Microbial reduction of 3,5-dioxo-6-(benzyloxy) hexanoic acid, ethyl-ester. Enzyme Microb Technol 15:1014–1021
Peters J (1998) Dehydrogenases – characteristics, design of reaction conditions, and applications. In: Rehm HJ, Reed G (eds) Biotechnology Vol 8a: Biotransformations I, 2nd edn. Wiley-VCH Verlag GmbH, Weinheim, pp 393–460
Pohl M, Liese A (2006) Industrial processes using lyases for C–C, C–N and C–O bond formation. In: Patel RN (ed) Biocatalysis in the pharmaceutical and biotechnology industries. Taylor & Francis, New York, pp 661–676
Raunio R, Lilius EM (1971) Effect of dithionite on enzyme activities in vivo. Enzymologia 40:360–368
Reetz MT, Zonta A, Schimossek K, Liebeton K, Jaeger KE (1997) Erzeugung enantioselektiver biokatalysatoren für die organische chemie durch in-vitro-evolution. Angew Chem 109:2961–2963 (also in Angew Chem Int Ed Engl 36:2830–2832)
Rickus JL, Tobin AJ, Zink JI, Dunn B (2002) Photochemical enzyme co-factor regeneration: towards continuous glutamate monitoring with a sol–gel optical biosensor. Mater Res Soc Symp Proc 723:155–160
Rissom S, Beliczey J, Giffels G, Kragl U, Wandrey C (1999) Asymmetric reduction of acetophenone in membrane reactors: comparison of oxazaborolodine and alcohol dehydrogenase catalyzed processes. Tetrahedron Asymmetry 10:923–928
Rosche B, Sandford V, Breuer M, Hauer B, Rogers PL (2002) Enhanced production of R-phenylacetylcarbinol (R-PAC) through enzymatic biotransformation. J Mol Catal B Enzym 19–20:109–115
Rosen TC, Daußmann T, Stohrer J (2004) Bioreduction forms optically active 3-hydroxyesters. Speciality Chemicals Magazine, April, pp 39–40
Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts M, Witholt B (2001) Industrial biocatalysis today and tomorrow. Nature 409:258–268
Schmidt E, Ghisalba O, Gygax D, Sedelmeier G (1992) Optimization of a process for the production of (R)-2-hydroxy-4-phenylbutyric acid—an intermediate for inhibitors of angiotensin converting enzyme. J Biotechnol 24:315–327
Schröder I, Steckhan E, Liese A (2003) In situ NAD+ regeneration using 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) as an electron transfer mediator. J Electroanal Chem 541:109–115
Schubert T, Hummel W, Müller M (2002) Highly enantioselective preparation of multifunctionalized propargylic building blocks. Angew Chem 114:656–659 (also in Angew Chem Int Ed 41:634–637)
Seelbach K, Riebel B, Hummel W, Kula MR, Tishkov VI, Egorov AM, Wandrey C, Kragl U (1996) A novel, efficient regenerating method of NADPH using a new formate dehydrogenase. Tetrahedron Lett 31:1377–1380
Simon H, Bader J, Gunther H, Neumann S, Thanos J (1985) Chirale verbindungen durch biokatalytische reduktionen. Angew Chem 97:541–555 (also in Angew Chem Int Ed Engl 24:539–553)
St. Clair N, Wang YF, Margolin AL (2000) Cofactor-bound cross-linked enzyme crystals (CLEC) of alcohol dehydrogenase. Angew Chem 112:388–391 (also in Angew Chem Int Ed 39:380–383)
Stark D, von Stockar U (2003) In situ product removal (ISPR) in whole cell biotechnology during the last twenty years. In: Scheper T (ed) Advances in biochemical engineering/biotechnology (vol 80). Springer, Berlin Heidelberg New York, pp 149–175
Steckhan E, Herrmann S, Ruppert R, Thömmes J, Wandrey C (1990) Kontinuierliche Erzeugung von NADH aus NAD+ und Formiat mit einem molekulargewichtsvergrößerten Homogenkatalysator in einem Membranreaktor. Angew Chem 102:445–447 (also in Angew Chem Int Ed Engl 29:388–390)
Steckhan E, Herrmann S, Ruppert R, Dietz E, Frede M, Spika E (1991) Analytical study of a series of substituted (2,2′-bipyridyl) (pentamethylcyclopentadienyl)rhodium and -iridium complexes with regard to their effectiveness as redox catalysts for the indirect electrochemical and chemical reduction of NAD(P)+. Organometallics 10:1568–1577
Stillger T, Bönitz M, Filho MV, Liese A (2002) Überwindung von thermodynamischen Limitierungen in substratgekoppelten Cofaktorregenerierungsverfahren. Chem Ing Tech 74:1035–1039
Stillger T, Pohl M, Wandrey C, Liese A (2006) Reaction engineering of benzaldehyde lyase from Pseudomonas fluorescens catalyzing enantioselective C–C bond formation. Org Process Res Dev 10:1172–1177
Takors R (2004) Ganzzell-ISPR-Prozessentwicklung: chancen und risiken. Chem Ing Tech 76:1857–1864
Tao JH, McGee K (2002) Development of a continuous enzymatic process for the preparation of (R)-3-(4-fluorophenyl)-2-hydroxy propionic acid. Org Process Res Dev 6:520–524
Tishkov VI, Galkin AG, Fedorchuk VV, Savitsky PA, Rojkova AM, Gieren H, Kula MR (1999) Pilot scale production and isolation of recombinant NAD+- and NADP+-specific formate dehydrogenases. Biotechnol Bioeng 64:187–193
Trivedi A, Heinemann M, Spieß AC, Daußmann T, Büchs J (2005) Optimization of adsoptive immobilization of alcohol dehydrogenase. J Biosci Bioeng 99:340–347
Trivedi A, Spieß AC, Daußmann T, Büchs J (2006) Study on mesophilic and thermophilic alcohol dehydrogenases in gas-phase reaction. Biotechnol Prog 22:454–458
van Beilen JB, Duetz WA, Schmid A, Witholt B (2003) Practical issues in the application of oxygenases. Trends Biotechnol 21:170–177
Van Rantwijk F, Lau RM, Sheldon RA (2003) Biocatalytic transformations in ionic liquids. Trends Biotechnol 21:131–138
Villela M, Stillger T, Müller M, Liese A, Wandrey C (2003) Is logP a convenient criterion to guide the choice of solvents for biphasic enzymatic reactions? Angew Chem 115:3101–3104 (also in Angew Chem Int Ed 42:2993–2996)
von Scala C, Fässler P, Gerla J, Maus E (2005) Kontinuierliche herstellung von kosmetischen fettsäureestern mittels reaktivdestillation und pervaporation. Chem Ing Tech 77:1809–1813
Vrtis JM, White AK, Metcalf WW, van der Donk WA (2002) Phosphite dehydrogenase: a versatile cofactor-regeneration enzyme. Angew Chem 114:3391–3393 (also in Angew Chem Int Ed 41:3257–3259)
Vuorilehto K, Lütz S, Wandrey C (2004) Indirect electrochemical reduction of nicotinamide coenzymes. Bioelectrochemistry 65:1–7
Wagenknecht PS, Penney JM, Hembre RT (2003) Transition-metal-catalyzed regeneration of nicotinamide coenzymes with hydrogen. Organometallics 22:1180–1182
Wallner SR, Bauer M, Würdemann C, Wecker P, Glöckner FO, Faber K (2005) Highly enantioselective sec-alkyl sulfatase activity of the marine planctomycete Rhodopirellula baltica shows retention of configuration. Angew Chem 117:6539–6542 (also in Angew Chem Int Ed 44:6381–6384)
Walsh C (1980) Flavin coenzymes: at the crossroads of biological redox chemistry. Acc Chem Res 13:148–155
Wandrey C (2004) Biochemical reaction engineering for redox reactions. Chem Rec 4:254–265
Weckbecker A, Hummel W (2004) Improved synthesis of chiral alcohols with Escherichia coli cells co-expressing pyridine nucleotide transhydrogenase, NADP+-dependent alcohol dehydrogenase and NAD+-dependent formate dehydrogenase. Biotechnol Lett 26:1739–1744
Weckbecker A, Hummel W (2005) Glucose dehydrogenase for the regeneration of NADPH and NADH. In: Barredo JL (ed) Microbial enzymes and biotransformations. Humana, Totowa, NJ, pp 225–238
Weuster-Botz D, Paschold H, Striegel B, Gieren H, Kula MR, Wandrey C (1994) Continuous computer controlled production of formate dehydrogenase (FDH) and isolation on a pilot scale. Chem Eng Technol 17:131–137
Wichmann R, Vasic-Racki D (2005) Cofactor regeneration at the lab scale. In: Kragl U (ed) Advances in biochemical engineering/biotechnology (vol 92). Springer, Berlin Heidelberg New York, pp 225–260
Willner I, Maidan R, Shapira M (1990) Thermal and photochemical regeneration of nicotinamide cofactors and a nicotinamide model Compound Using a water-soluble rhodium phosphine catalyst. J Chem Soc Perkin Trans 2:559–564
Wolberg M, Hummel W, Wandrey C, Müller M (2000) Highly regio- and enantioselective reduction of 3,5-dioxocarboxylates. Angew Chem 112:4476–4478 (also in Angew Chem Int Ed 39:4306–4308)
Wong CH, Daniels L, Orme-Johnson WH, Whitesides GM (1981) Enzyme-catalyzed organic synthesis: NAD(P)H regeneration using dihydrogen and the hydrogenase from Methanobacterium thermoautotrophicum. J Am Chem Soc 103:6227–6238
Yuan R, Watanabe S, Kuwabata S, Yoneyama H (1997) Asymmetric electroreduction of ketone and aldehyde derivatives to the corresponding alcohols using alcohol dehydrogenase as an electrocatalyst. J Org Chem 62:2494–2499
Zelinski T, Liese A, Wandrey C, Kula MR (1999) Asymmetric reductions in aqueous media: enzymatic synthesis in cyclodextrin containing buffers. Tetrahedron Asymmetry 10:1681–1687
Author information
Authors and Affiliations
Corresponding author
Additional information
Katja Goldberg and Kirsten Schroer did equally contribute to this review.
Rights and permissions
About this article
Cite this article
Goldberg, K., Schroer, K., Lütz, S. et al. Biocatalytic ketone reduction—a powerful tool for the production of chiral alcohols—part I: processes with isolated enzymes. Appl Microbiol Biotechnol 76, 237–248 (2007). https://doi.org/10.1007/s00253-007-1002-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1002-0